IBP1218_09 CORROSION BY CONCENTRATED SULFURIC ACID IN CARBON STEEL PIPES AND TANKS – STATE OF THE ART Zehbour Panossian , Neus
a) What is the normality of a 96 per cent solution of H(2)SO(4) of specific gravity 1.84 ? (b) How many mL of 96 per cent sulphuric acid solution is necessary to

Concentrated aqueous sulphuric acid is `98% H_(2)SO_(4)` by mass and has a density of `1.80 g mL - YouTube

What is the molarity of concentrated sulfuric acid if it is 96% by mass H2so4 and has a density of 1.84g/mL? - Quora
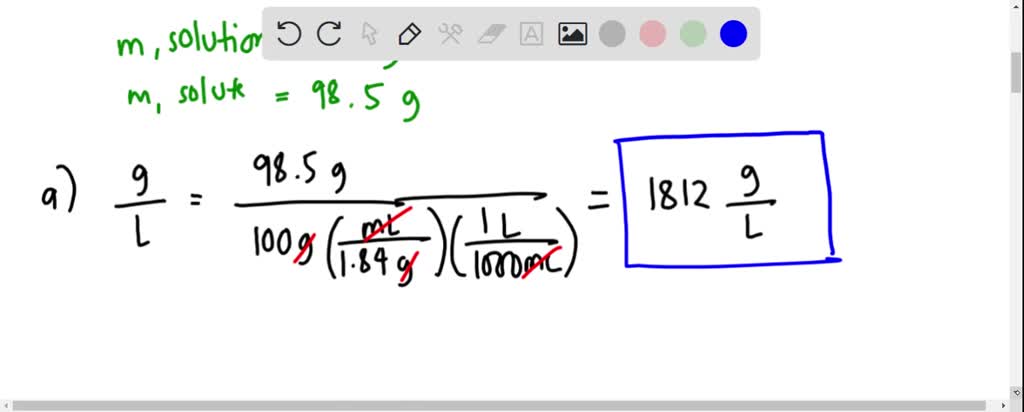
SOLVED: The concentrated sulfuric acid available in laboratories is approximately 98.6% by mass and its density is 1.871 g / mL. (MM H2SO4 = 98.08 g / mol;) Describe how you would
Sulphuric acid, 2.5 l, glass, CAS No. 7664-93-9 | Determination of Viscosity | Analysis of Food | Inorganic & Analytical Reagents | Chemicals | Carl Roth - International
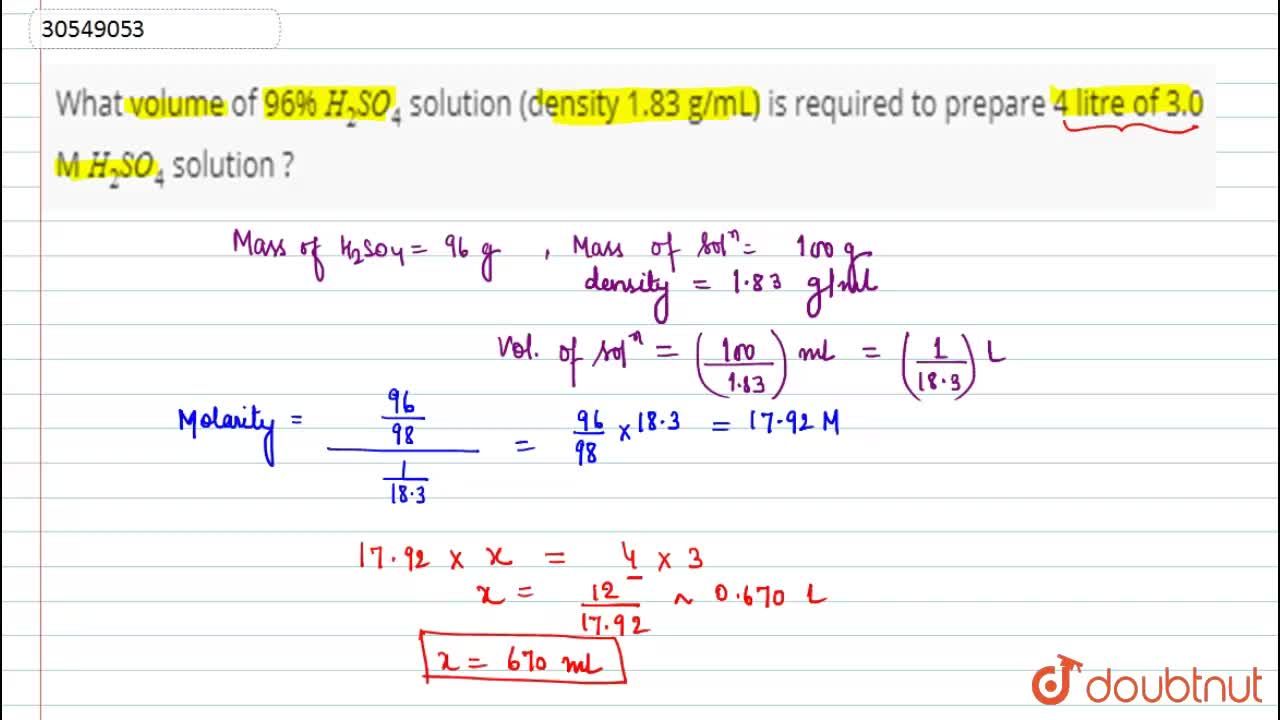
What volume of 96% H(2)SO(4) solution (density 1.83 g/mL) is required to prepare 4 litre of 3.0 M H(2)SO(4) solution ?

What is the molarity of concentrated sulfuric acid if it is 96% by mass H2so4 and has a density of 1.84g/mL? - Quora

What is the molarity of concentrated sulfuric acid if it is 96% by mass H2so4 and has a density of 1.84g/mL? - Quora

SOLVED: The density of 96% solution of sulphuric acid is equal to 1.84 g/mL. 100 mL of this solution are mixed with 400 mL of distilled water. Calculate the percentage, molarity, normality (
What volume of concentrated H2SO4 (density = 1.84g/mL and 96% purity) would be required to prepare 500mL of a 0.2000M H2SO4 solution? - Quora
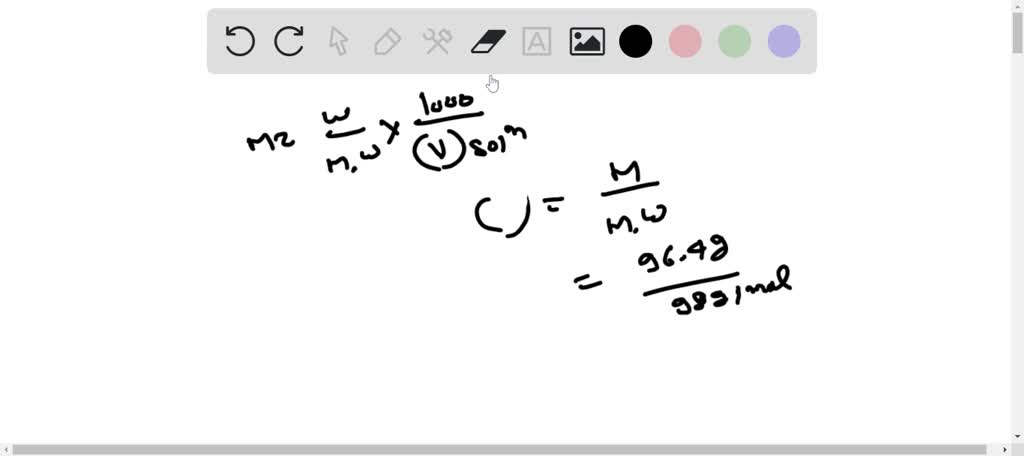
SOLVED: A sample of commercial sulfuric acid is 96.4 % H2SO4 by mass, and its specific gravity is 1.84. Calculate the molarity of this sulfuric acid solution. Molecular weight of sulfuric acid
What is the volume of concentrated H2SO4 of specific gravity 1.84 and containing 98% H2SO4 by weights that would contain 40 gm of pure H2SO4? - Quora