PDF) Dissociation Constant of Acetic Acid in (N,N-Dimethylformamide + Water) Mixtures at the Temperature 298.15 K

Calculate heat of dissociation for acetic acid from the following data: CH(3)COOH +NaOH rarr CH(3)COONa +H(2)O DeltaH = - 13.2 kcal H^(o+) +overset(Theta)Ohrarr H(2)O, DeltaH =- 13.7 kcal Also calculate heat of

The enthalpy of neutralization of acetic acid and sodium hydroxide is - 55.4 kJ. What is the enthalpy of ionisation of acetic acid?

a) Calculate heat of dissociation for Acetic acid from the following data: CH3COOH + NaOH⟶ CH3COONa + H2O .... Δ H = - 13.2 Kcal H^⊕ + OH⟶ H2O; .... Δ H = -
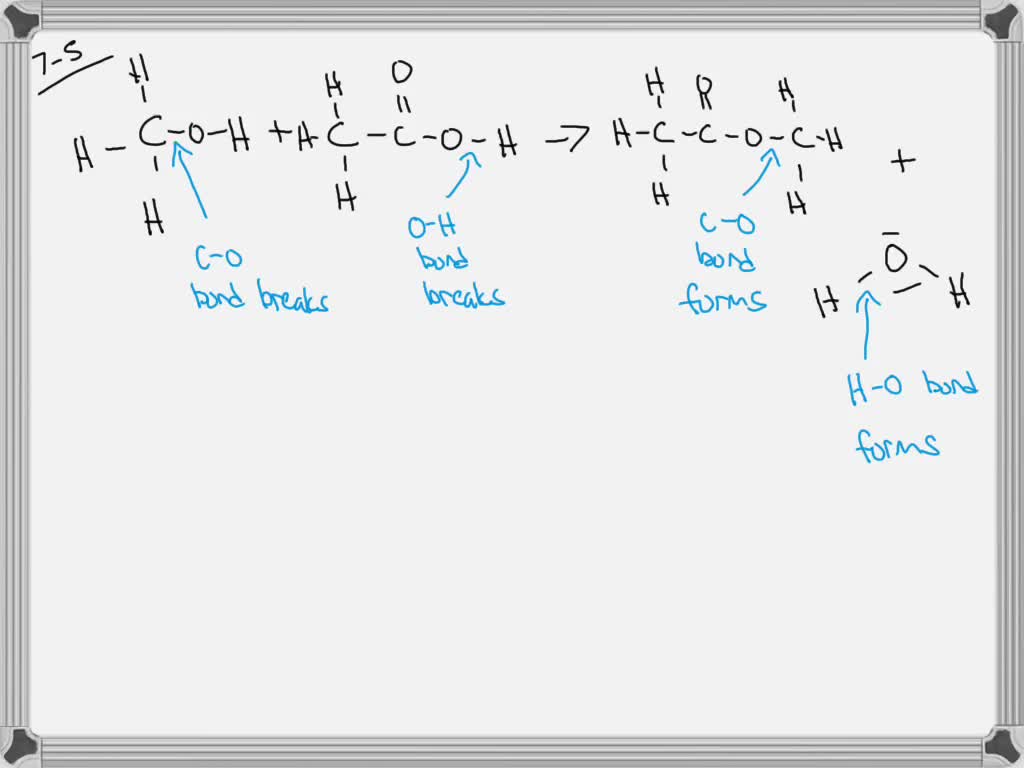
SOLVED:Enthalpy of dissociation (in kJ mol^-1 ) of acetic acid obtained from the Expt.2 is (a) 1.0 (b) 10.0 (c) 24.5 (d) 51.4
Heat of dissociation of CH3COOH is 0.005 kcal g^–1, hence enthalpy change when 1 mole of Ca(OH)2 is completely - Sarthaks eConnect | Largest Online Education Community

The ionization constant of acetic acid `1.74xx10^(-5)`. Calculate the degree of dissociation of ... - YouTube

equilibrium - Why is the dissociation reaction of acetic acid in water initially endothermic and then exothermic? - Chemistry Stack Exchange

The enthalpy of neutralization of acetic acid and sodium hydroxide is - 55.4 kJ. What is the enthalpy of ionisation of acetic acid?

The enthalpy formation of H2O (l) is - 285.7 kJ mol^- 1 and enthalpy of neutralization of strong acid and base is - 57.6 kJ mol^- 1 . what is the enthalpy of formation of OH^- 1 ions
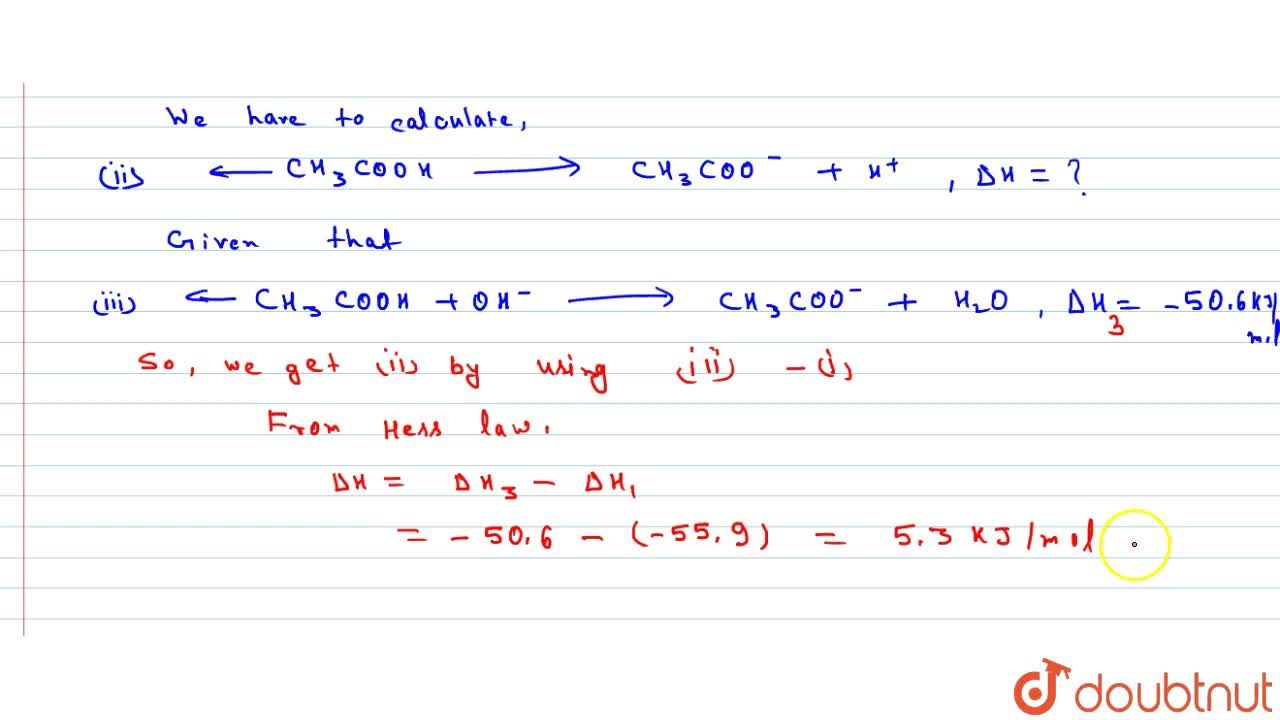
Enthalpy of neutralisation of acetic acid by NaOh is -50.6 kJ mol^(-1). Calculate DeltaH for ionisation of CH(3)COOH. Given. The heat of neutralisation of a strong acid with a strong base is -
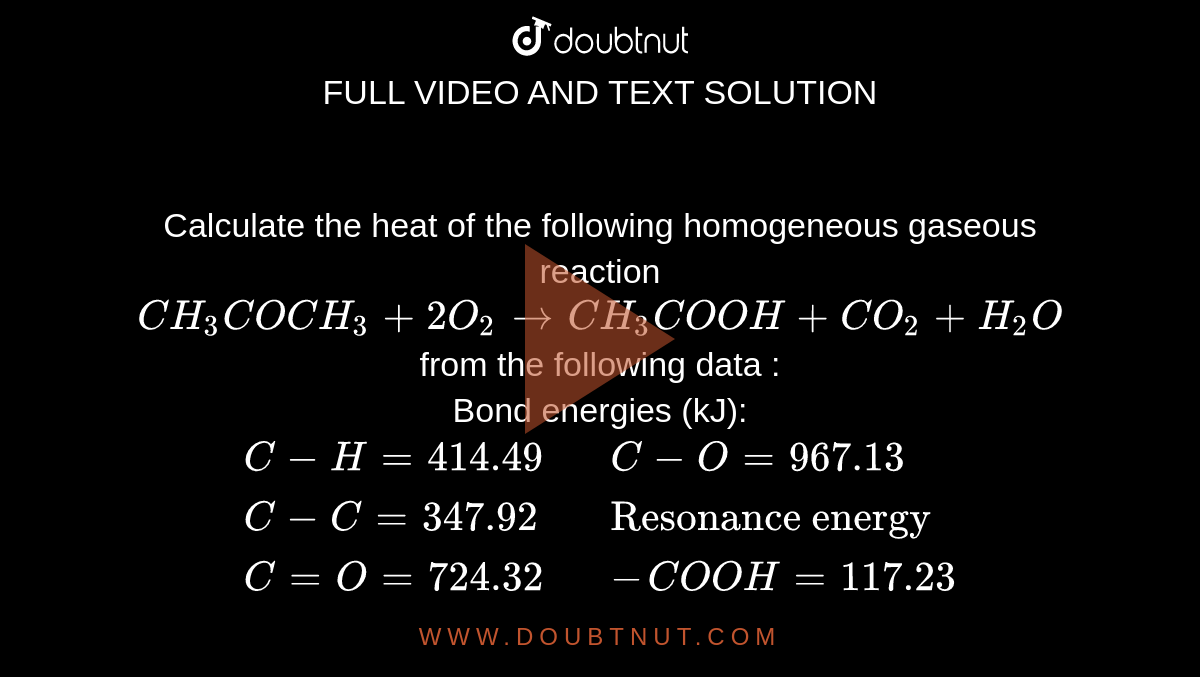
Calculate the resonance energy in CH(3)COOH from the following data if the observed heat of formation of CH(3)COOH is -439.7 kJ. {:("Bond energy (kJ)",,"Heat of atomisation (kJ)"),(C-H=413,,C=716.7),(C-C=348,,H=218.0),(C=O=732,,O=249.1),(C-O=351 ...
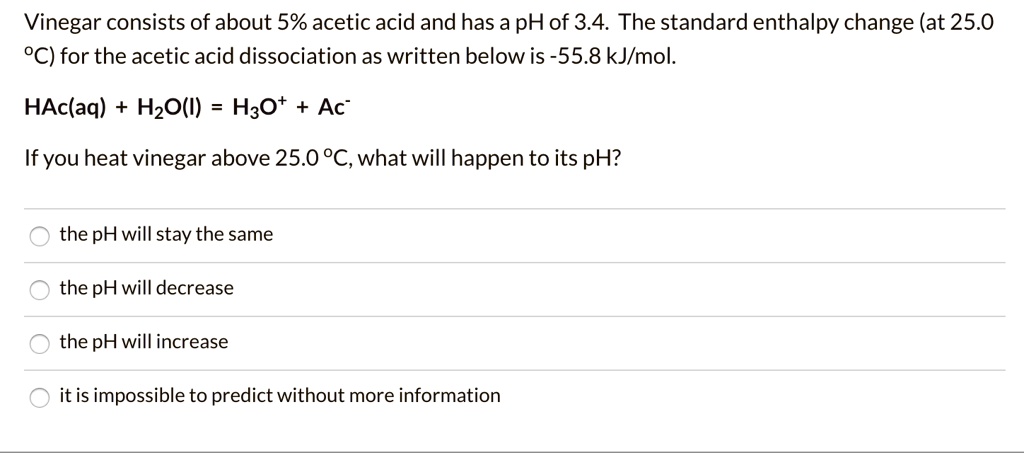
SOLVED: Vinegar consists of about 5% acetic acid and has a pH of 3.4. The standard enthalpy change (at 25.0 "C) for the acetic acid dissociation as written belowis-55.8 kJlmol. HAc(aq) HzO()
26. Heat of dissociation of acetic acid is 0.005 kcal per g hence enthalpy change when 1 mol of calcium hydroxide is completely neutrilised is?

Calculate heat of dissociation for acetic acid from the following data: `CH_(3)COOH +NaOH rarr C... - YouTube

SOLVED: what is the standard enthalpy of dissociation of acetic acid? H(C2H3O2) (aq) –> H+ (aq) + (C2H3O2)- (aq)

Bond dissociation energies for a series of acids: acetic, propanoic,... | Download Scientific Diagram