mass spectrum of ethanoic acid fragmentation pattern of m/z m/e ions for analysis and identification

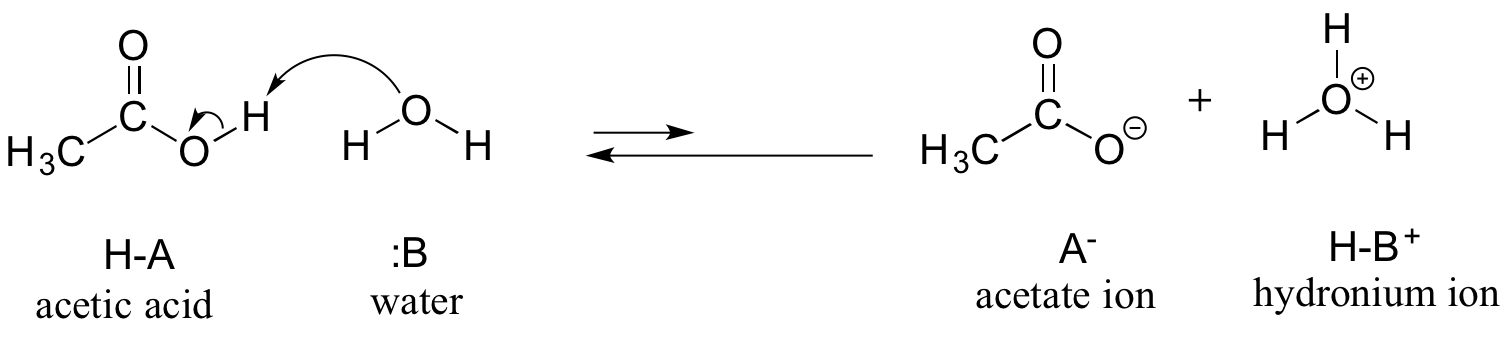
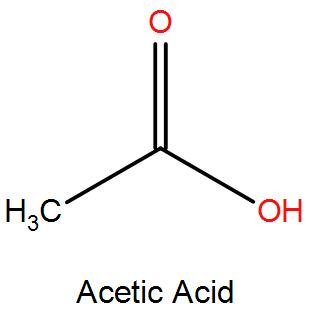
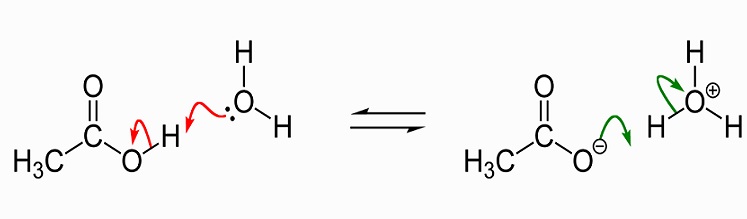
In terms of the bonds present, explain why acetic acid, CH_3CO_2H, contains two distinct types of carbon-oxygen bonds, whereas the acetate ion, formed by loss of a hydrogen ion from acetic acid,

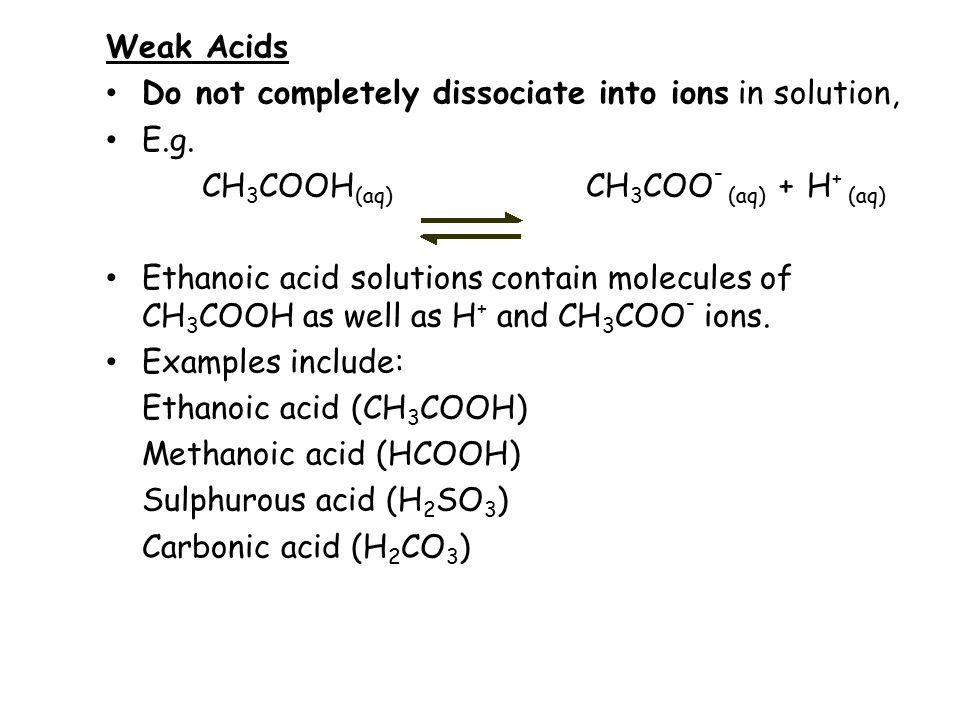
pKa for acetic acid is 4.74 . What should be the ratio of concentration of acetic acid and acetate ions to have a solution with pH 5.74 ?