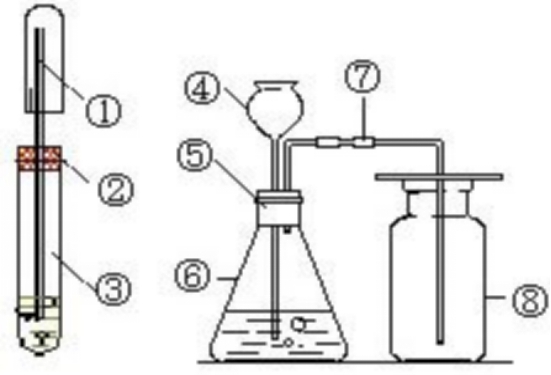
Manganese dioxide when reacts with hydrochloric acid forms manganese chloride, water and chlorine. - Sarthaks eConnect | Largest Online Education Community

Permanganate (VII) Ion, in basic solution, oxidize iodide ion I^ - to produce molecular iodine (I2) and manganese (IV) oxide (MnO2) . write a balanced ionic equation to represent this redox reaction.
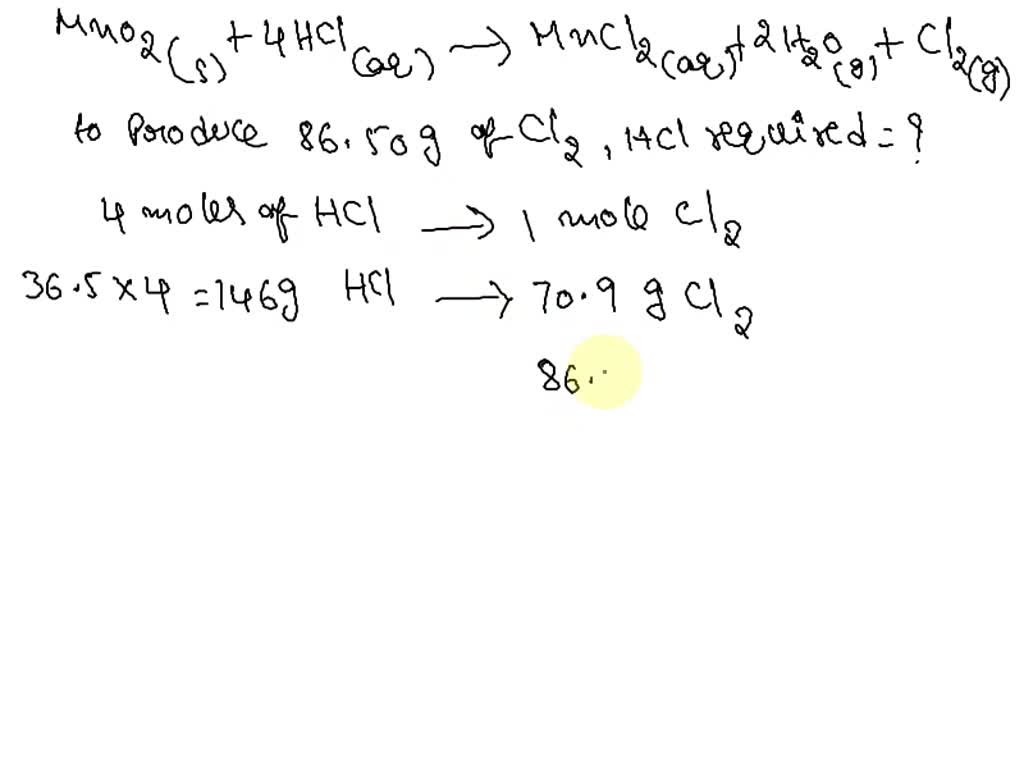
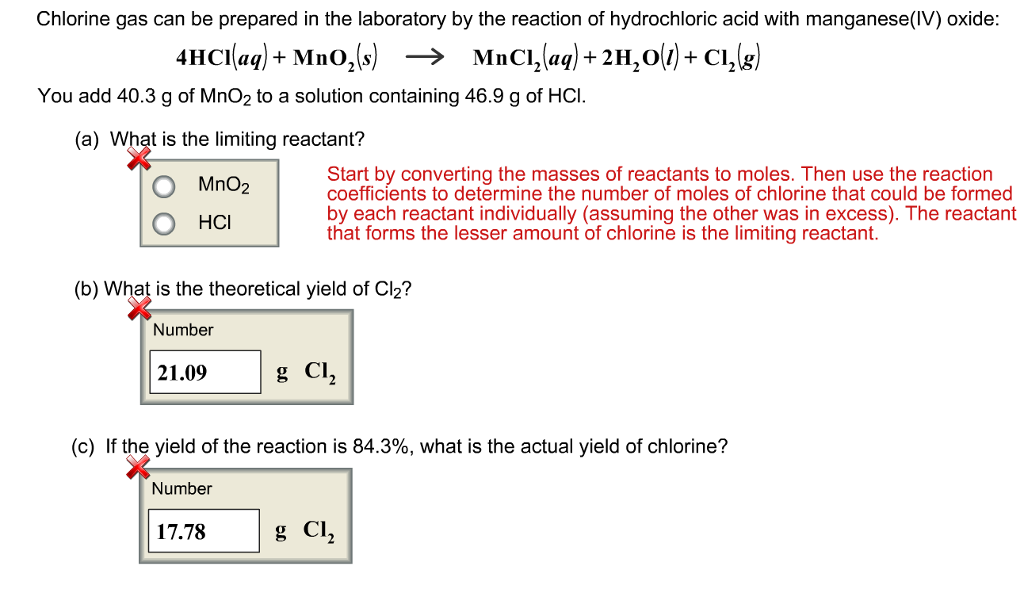
SOLVED: When manganese(IV) oxide (MnO2) reacts with hydrochloric acid (HCl), chlorine gas (Cl2) is produced as follows. MnO2(s) + 4 HCl(aq) → MnCl2(aq) + 2 H2O(g) + Cl2(g) How much HCl (in

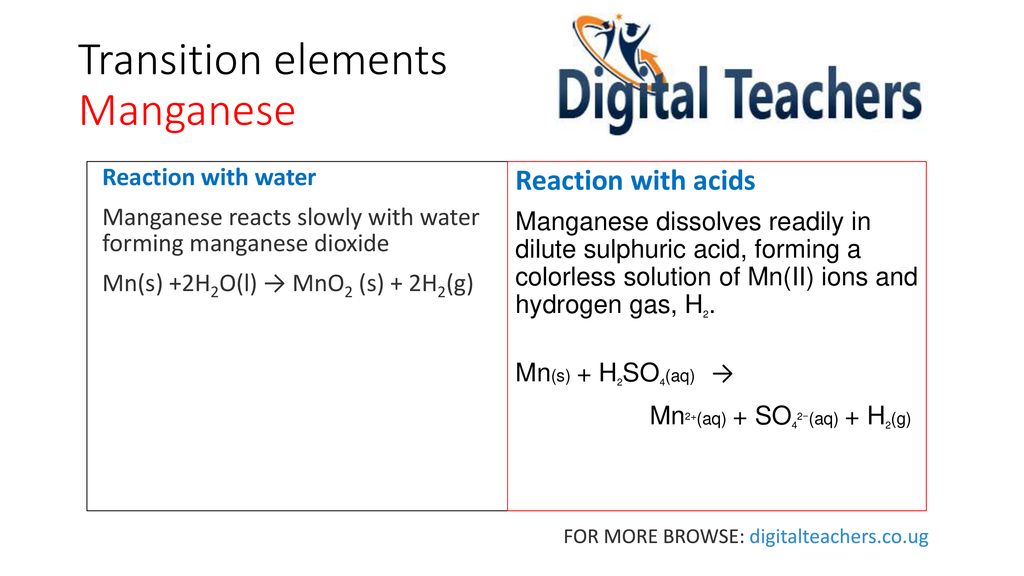
Write balanced equations for the following reactions: copper oxide and dilute hydrochloric acid. Manganese IV oxide and concentrated hydrochloric acid.litmus solutiona Name the experiment illustrated above.b Which property of hydrogen chloride is

Acids and Bases (3). Bases are the oxides or hydroxides of metals. Contains either oxide ions (O 2- ) or hydroxide ions (OH - ) BaseFormulaIons present. - ppt download

Manganese dioxide (MnO2) reacts with hydrochloric acid (HCl) to produce magnesium chloride (MgCl2), water (H2O), and chlorine (Cl2) gas. The chemical equation involved in the reaction is given as:MnO2 + 4 HCl

SOLVED: Chlorine gas was first prepared by Karl Scheele in 1774 by the reaction of sodium chloride, manganese(IV) oxide, and sulfuric acid. In addition to chlorine, the reaction produces water, sodium sulfate,

Optical absorption spectra for manganese dioxide powders obtained by... | Download Scientific Diagram

Understanding the Role of Manganese Dioxide in the Oxidation of Phenolic Compounds by Aqueous Permanganate | Environmental Science & Technology
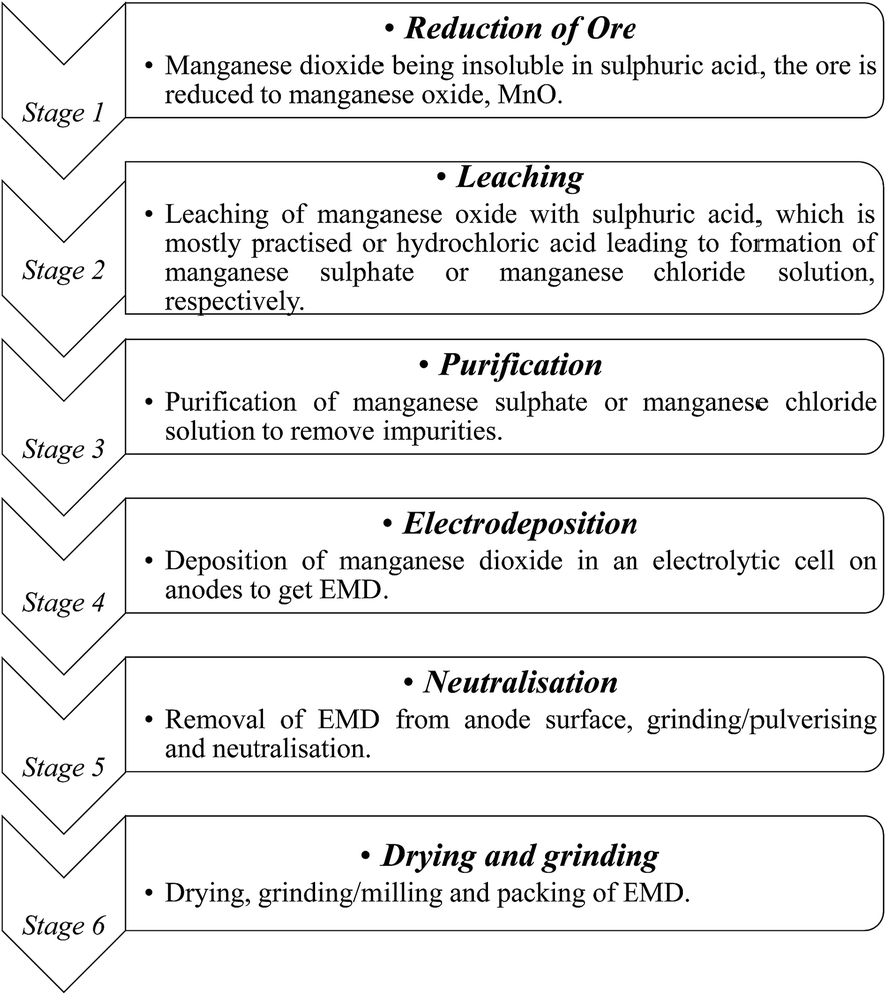
Electrolytic manganese dioxide (EMD): a perspective on worldwide production, reserves and its role in electrochemistry - RSC Advances (RSC Publishing) DOI:10.1039/C5RA05892A