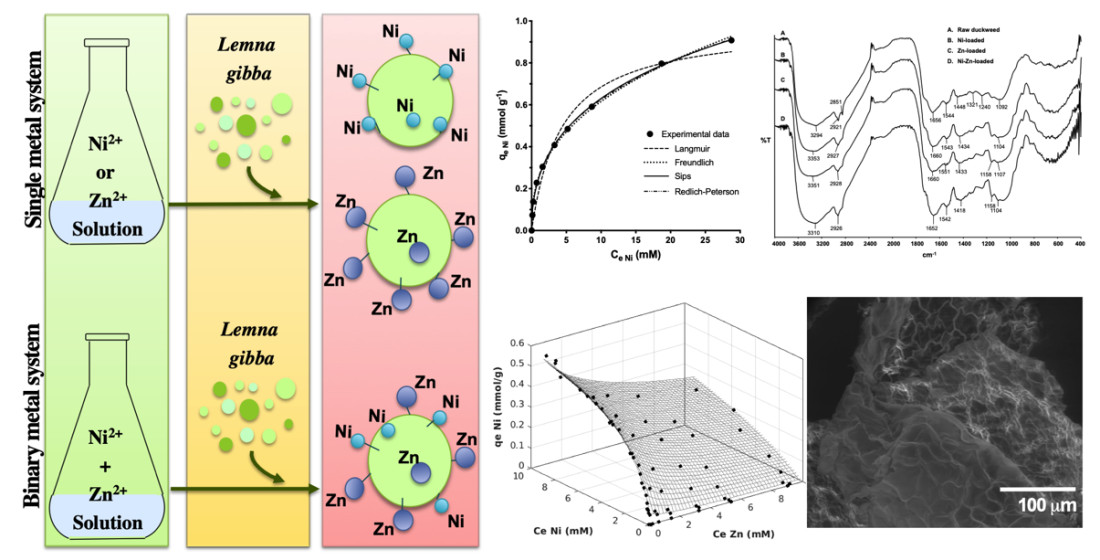
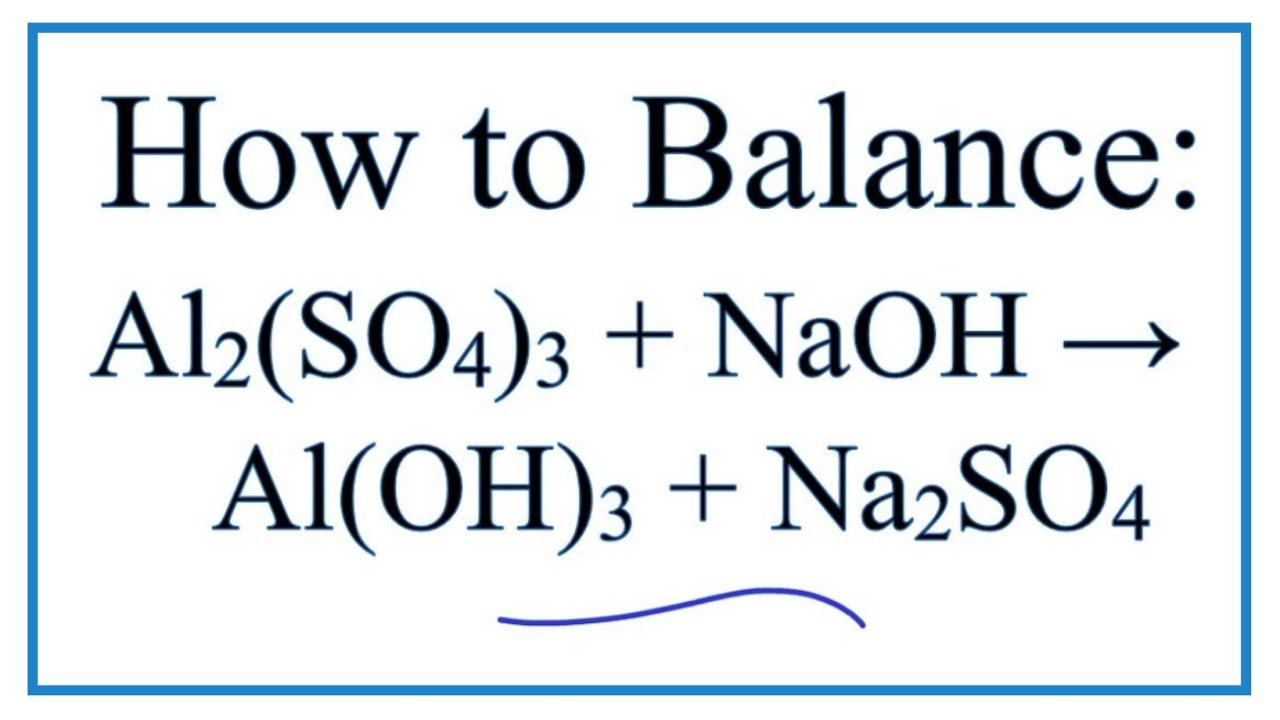
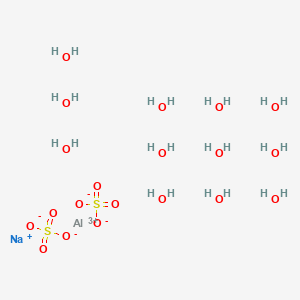
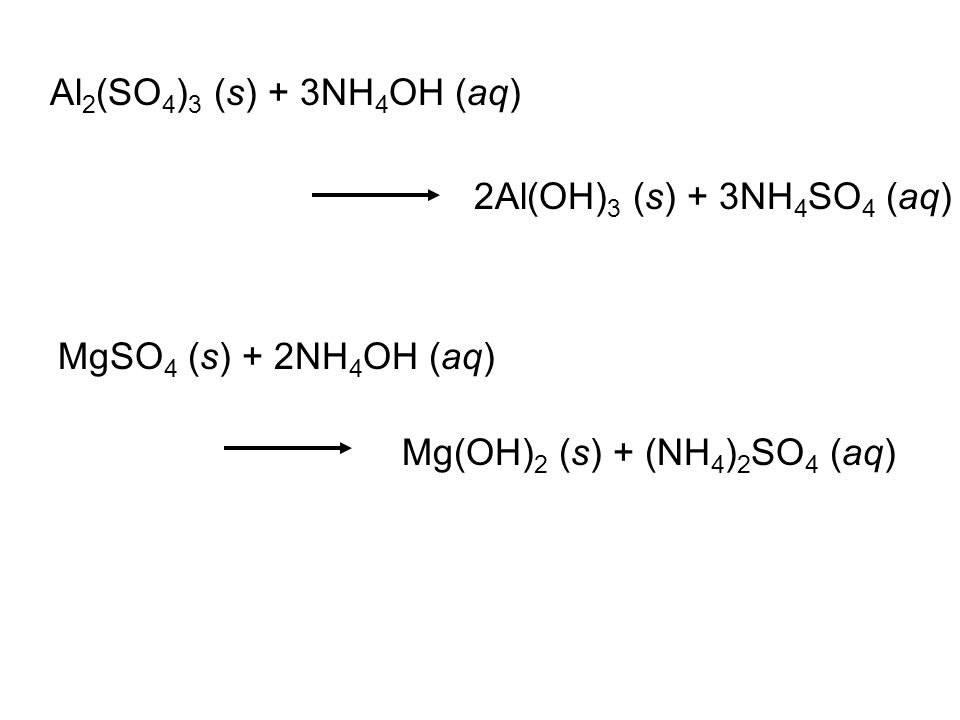
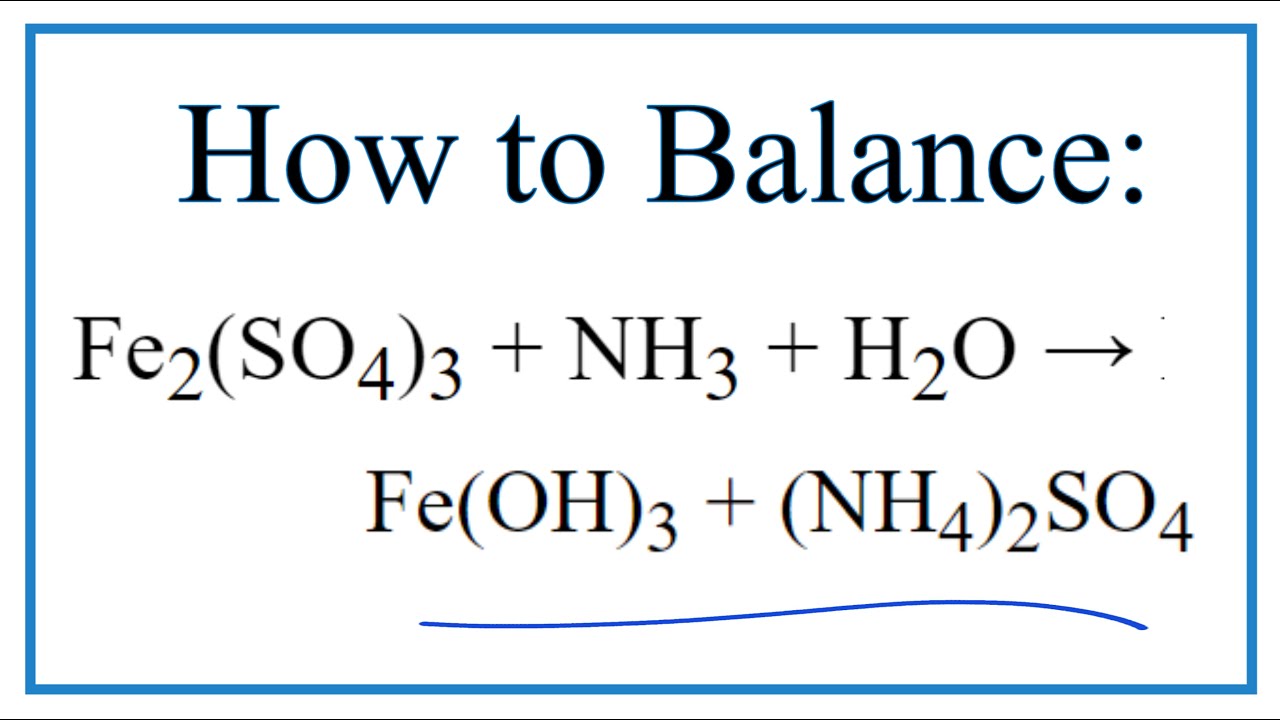
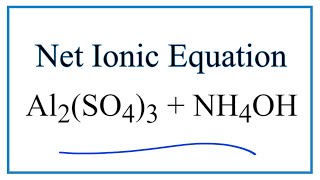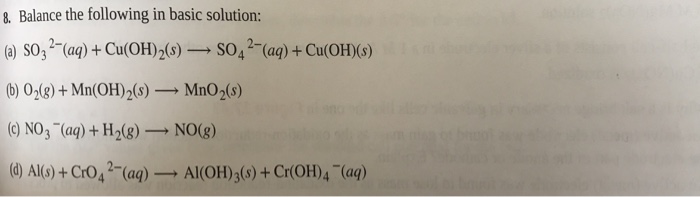PDF) The role of hydroxy aluminium sulfate minerals in controlling Al3+ concentration and speciation in acidic soils

Stereoselective Syntheses of l-Pipecolic Acid and (2S,3S)-3-Hydroxypipecolic Acid from a Chiral N-Imino-2-phenyl-1,2-dihydropyridine Intermediate | The Journal of Organic Chemistry

OneClass: write a balanced net ionic equation for A. dissolving of Ni (OH)2 in nitric acid. B. Ni 2+ ...
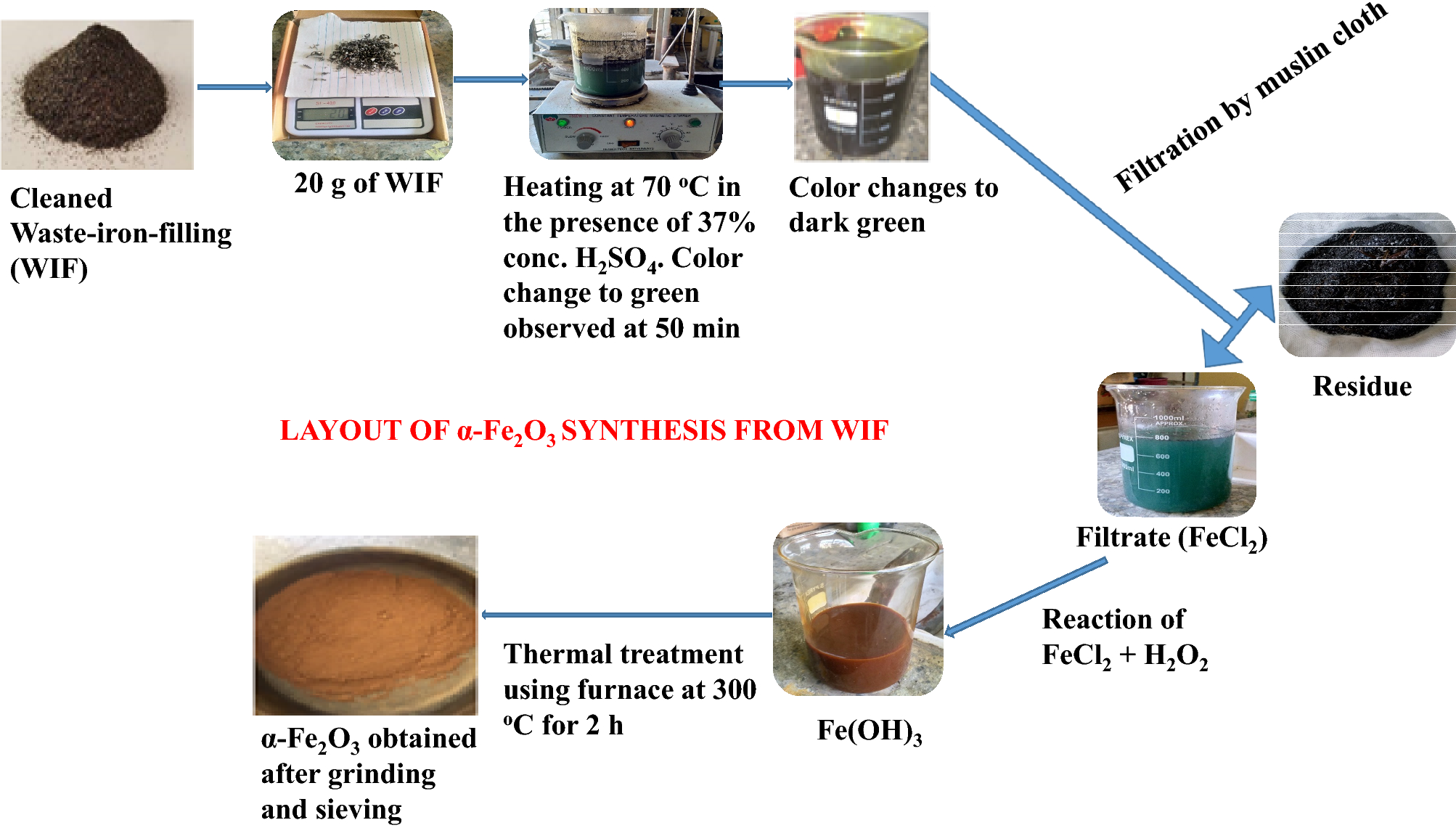
Nano-synthesis of solid acid catalysts from waste-iron-filling for biodiesel production using high free fatty acid waste cooking oil | Scientific Reports
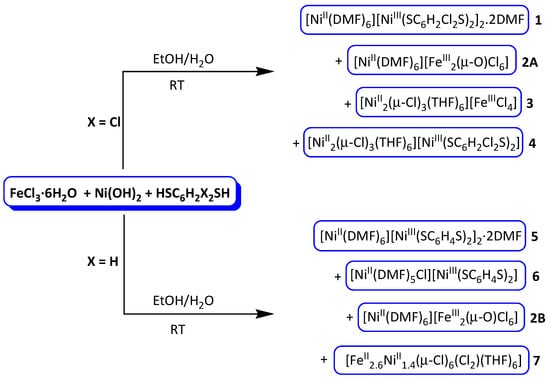
Molecules | Free Full-Text | Structural Study of the Compounds Formed in the Reactions of FeCl3·6H2O with Ni(OH)2 in the Presence of Dithiolenes HSRSH (R = C6H2Cl2 or C6H4)

Neutral Nickel Catalysts for Olefin Homo- and Copolymerization: Relationships between Catalyst Structures and Catalytic Properties | Chemical Reviews
![Given the IUPAC names of the following complex compounds. ltbr. i) [CoBr(NH(3)(5)]SO(4) [Fe(NH(3))(6)][Cr(CN)(6)] iii) Na(3)[FeCl(CN)(5)] iv) [Fe(OH)(H(2)O)(5)]^(2+) Given the IUPAC names of the following complex compounds. ltbr. i) [CoBr(NH(3)(5)]SO(4) [Fe(NH(3))(6)][Cr(CN)(6)] iii) Na(3)[FeCl(CN)(5)] iv) [Fe(OH)(H(2)O)(5)]^(2+)](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/30713886_web.png)
Given the IUPAC names of the following complex compounds. ltbr. i) [CoBr(NH(3)(5)]SO(4) [Fe(NH(3))(6)][Cr(CN)(6)] iii) Na(3)[FeCl(CN)(5)] iv) [Fe(OH)(H(2)O)(5)]^(2+)