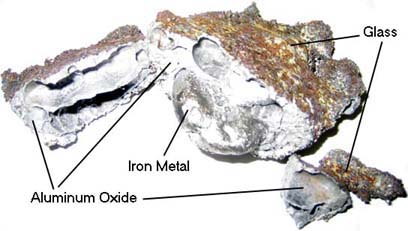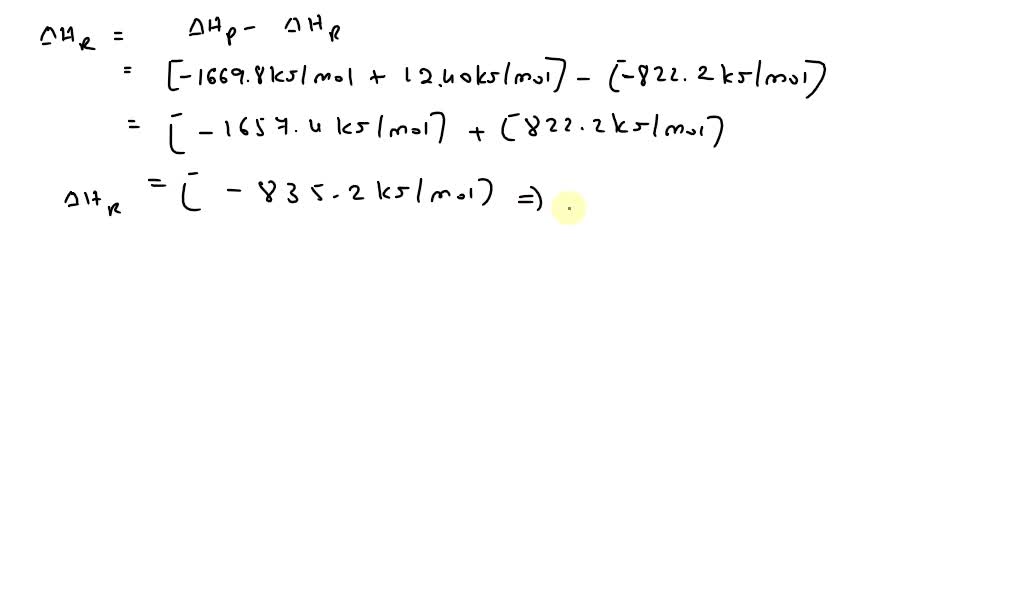
SOLVED: The thermite reaction involves aluminum and iron(III) oxide, 2Al(s) + Fe2O3(s) à Al2O3(s) + 2Fe(l) This reaction is highly exothermic and the liquid iron formed is used to weld metals. Calculate

DIY Thermite Required Materials Iron Oxide aka rust Powdered Aluminum (these are both available from paint stores) Magnesium strip or sparkler Protective mask and gloves Scale Bucket heavy duty plastic bag Container
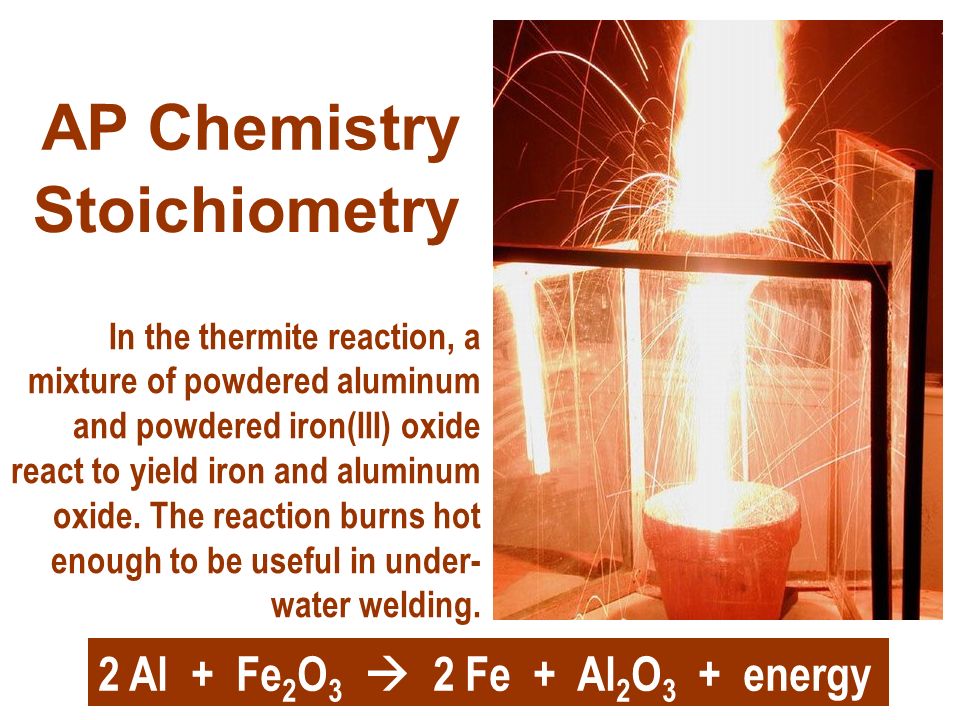
Stoichiometry AP Chemistry In the thermite reaction, a mixture of powdered aluminum and powdered iron(III) oxide react to yield iron and aluminum oxide. - ppt download
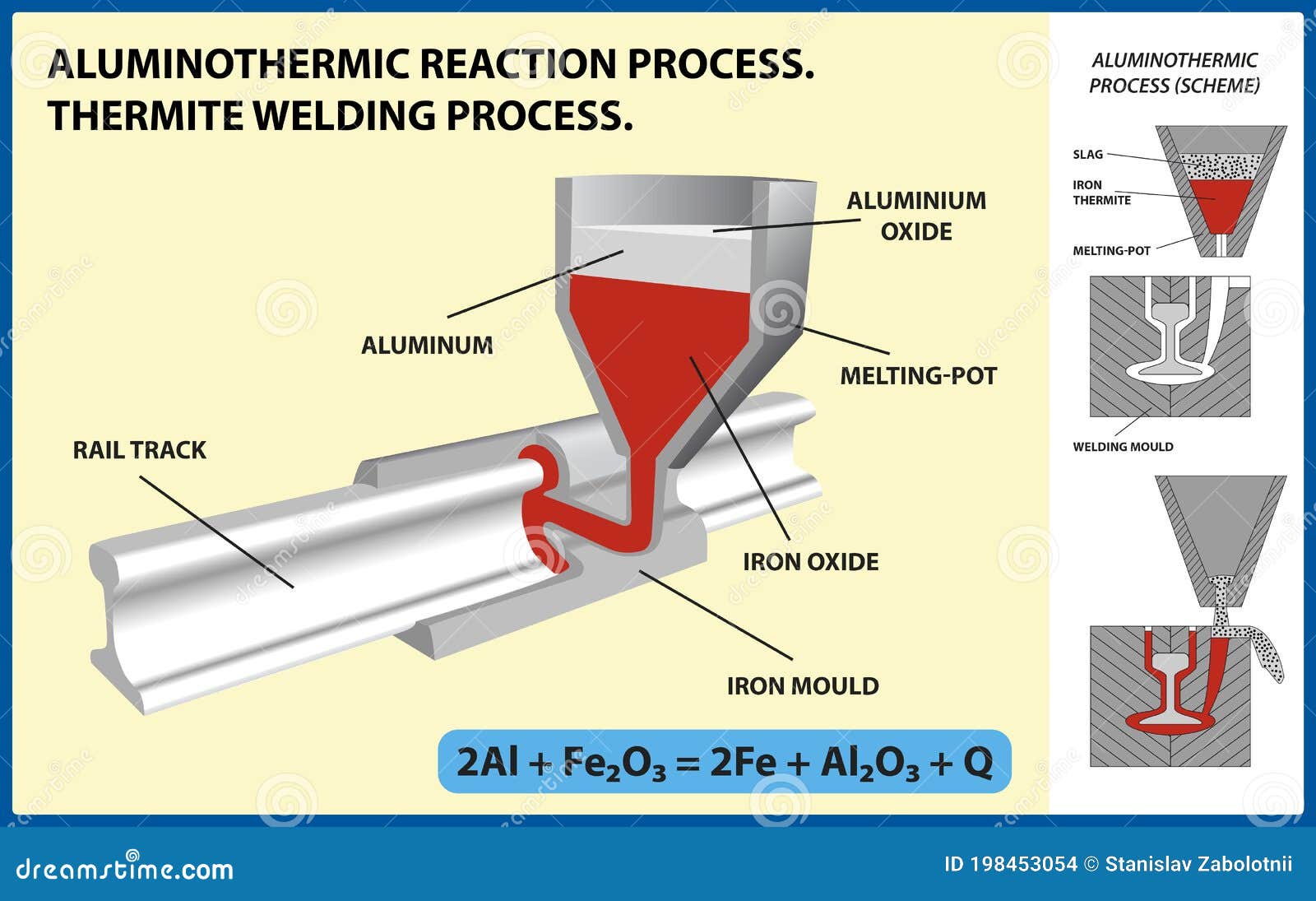
Aluminothermic Reaction Process. Thermite Welding Process. Vector Illustration Stock Vector - Illustration of evolved, mixture: 198453054
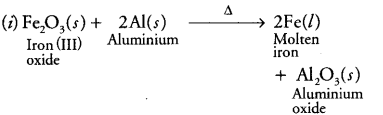
Write the balanced chemical equations for the following reactions and identify the type of reaction in each case. (i) In thermite reaction, iron (III) oxide reacts with aluminium and gives molten iron
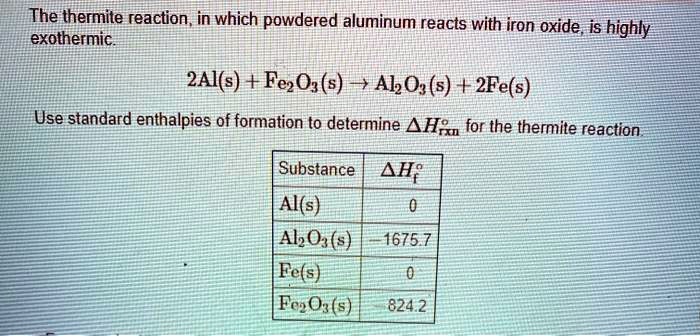
SOLVED: The thermite reaction, in which powdered aluminum reacts with iron oxide is highly exothermic 2Al(s) + Fe2Og (s) + AkO,(s) + 2Fe(s) Use standard enthalpies of formation to determine A H;n

SOLVED:In the thermite reaction, iron(III) oxide is reduced by aluminum to give molten iron. Fe2 O3(s)+2 Al(s) →2 Fe(ℓ)+Al2 O3(s) If you begin with 10.0 g of Fe2 O3 and 20.0 g
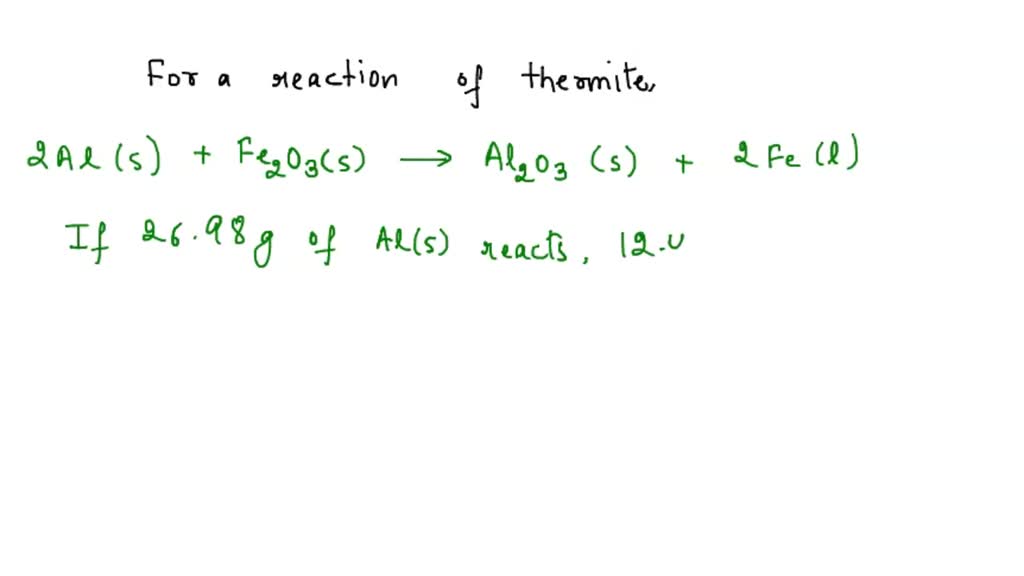
SOLVED: The thermite reaction involves aluminum and iron(III) oxide, 2Al(s) + Fe2O3(s) à Al2O3(s) + 2Fe(l) This reaction is highly exothermic and the liquid iron formed is used to weld metals. Calculate
![PDF] Mechanism for thermite reactions of aluminum/iron-oxide nanocomposites based on residue analysis | Semantic Scholar PDF] Mechanism for thermite reactions of aluminum/iron-oxide nanocomposites based on residue analysis | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/035f93a77120a19b7cb355ec122296f5319649ce/3-Figure1-1.png)

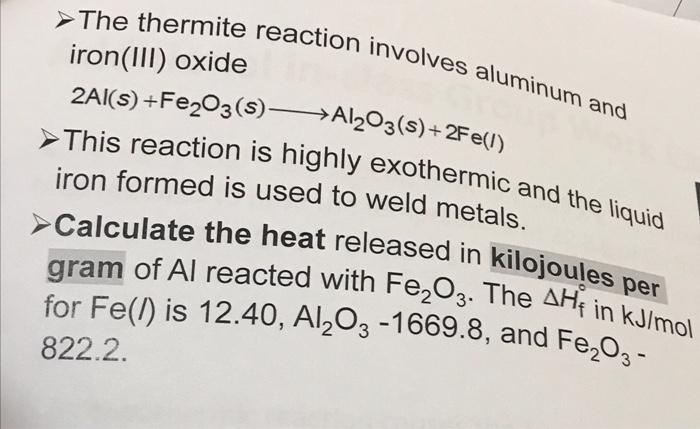











:max_bytes(150000):strip_icc()/GettyImages-578813868-5a787515a9d4f90036eff588.jpg)