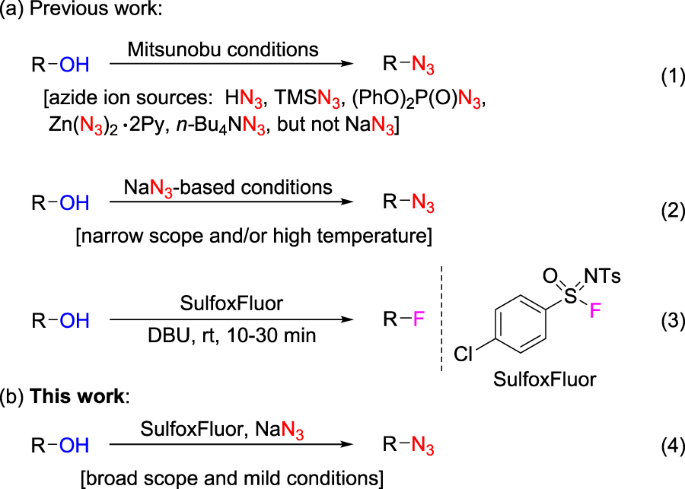
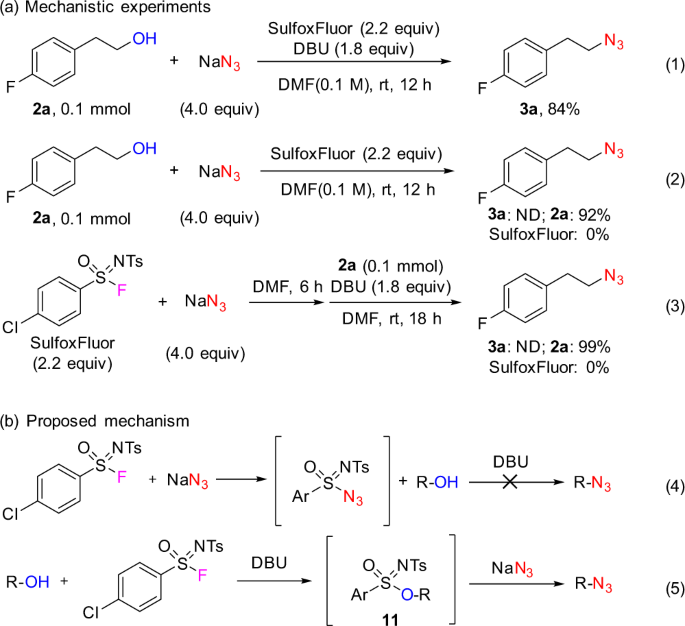
Mitsunobu Inversion of a Secondary Alcohol with Diphenylphosphoryl azide. Application to the Enantioselective Multikilogram Synthesis of a HCV Polymerase Inhibitor | Organic Process Research & Development

An exception to the normal Mitsunobu reaction with phenols; the formation of hydrazones from salicylaldehydes - ScienceDirect

An exception to the normal Mitsunobu reaction with phenols; the formation of hydrazones from salicylaldehydes - ScienceDirect

Azodicarbonyl dimorpholide (ADDM): an effective, versatile, and water-soluble Mitsunobu reagent - ScienceDirect

One‐Pot Mitsunobu Protocol to Access Ketene S,S‐/N,S‐Acetals at Room Temperature - Saha - 2021 - European Journal of Organic Chemistry - Wiley Online Library

The catalytic Mitsunobu reaction: a critical analysis of the current state-of-the-art - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C8OB01929K

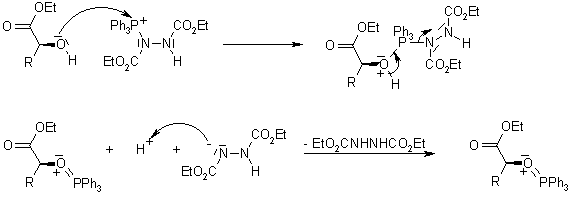
Where does the proton come from in a Mitsunobu azidation of secondary alcohol with DIAD/DPPA? | ResearchGate
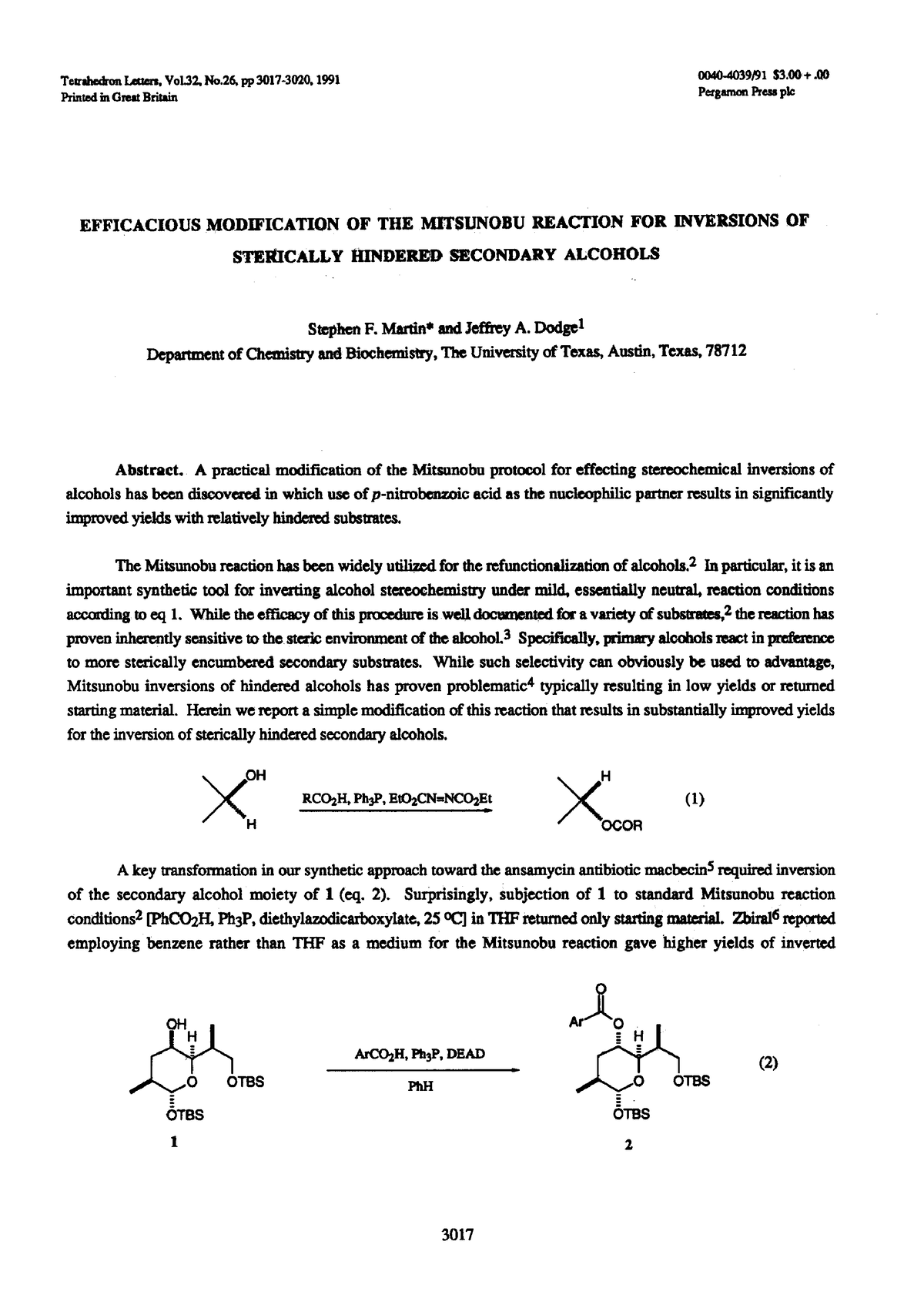
1-s2 - this paper is good - Tetrahcbn Lettax. Vol, No pi 3017-3020~ PrintedinGmatBribh oo4odo39/91 - Studocu

Where does the proton come from in a Mitsunobu azidation of secondary alcohol with DIAD/DPPA? | ResearchGate
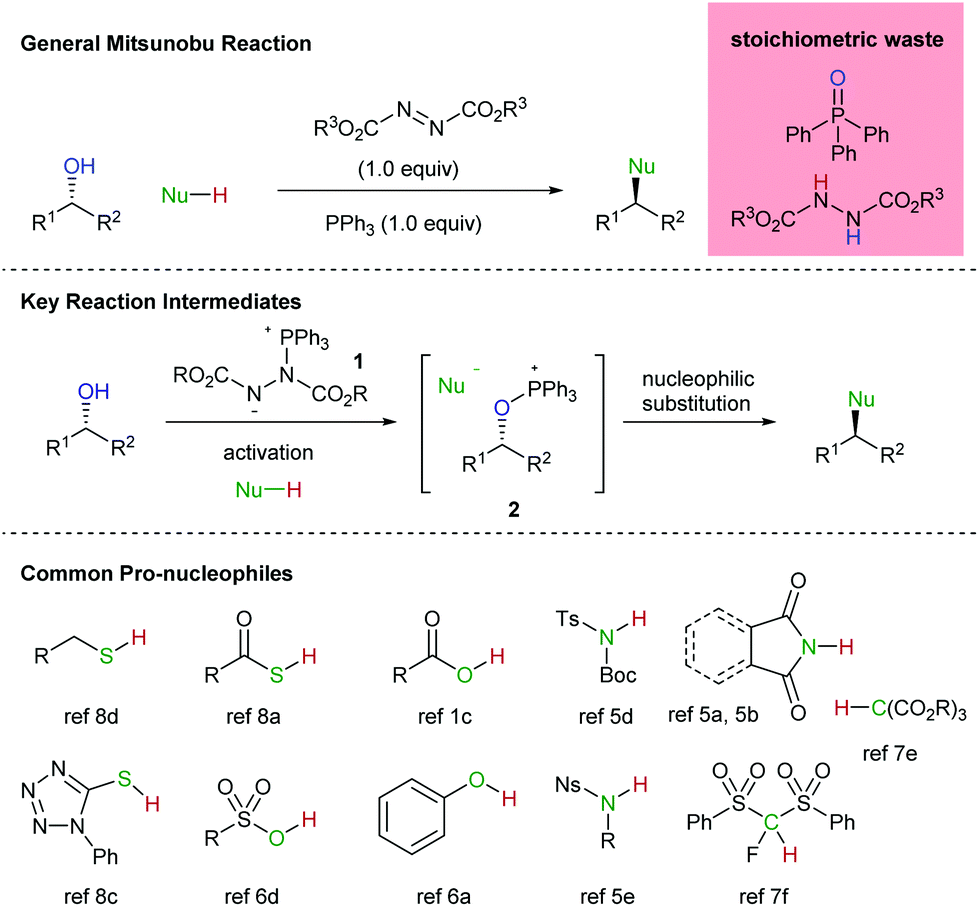
The catalytic Mitsunobu reaction: a critical analysis of the current state-of-the-art - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C8OB01929K
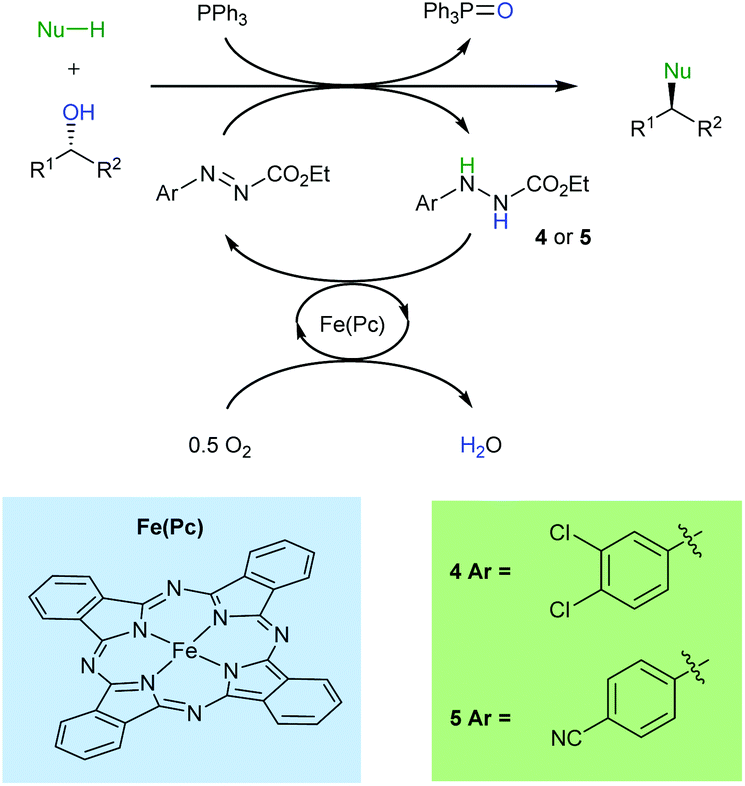
The catalytic Mitsunobu reaction: a critical analysis of the current state-of-the-art - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C8OB01929K

Base catalyzed Mitsunobu reactions as a tool for the synthesis of aryl sec-alkyl ethers - ScienceDirect
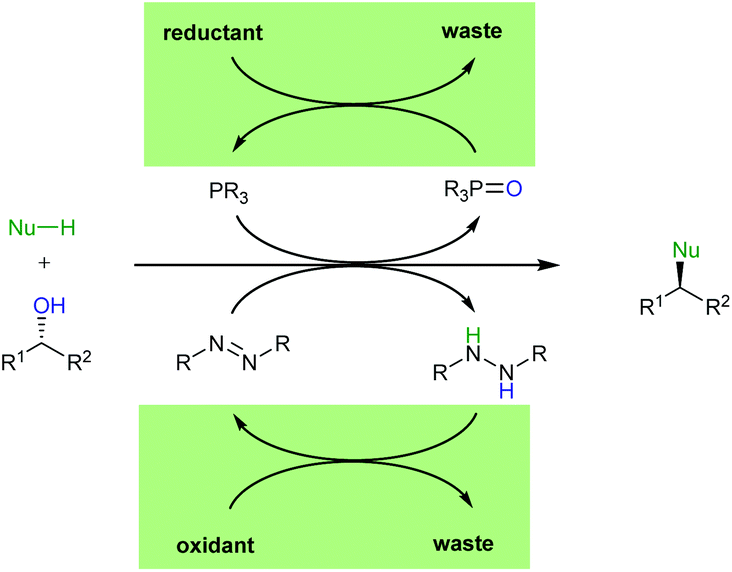
The catalytic Mitsunobu reaction: a critical analysis of the current state-of-the-art - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C8OB01929K

Cutting edge of diphenyl phosphorazidate (DPPA) as a synthetic reagent – A fifty-year odyssey - Vapourtec

Diversity-Oriented Approach to N-Heterocyclic Compounds from α-Phenyl-β-enamino Ester via a Mitsunobu-Michael Reaction Sequence | The Journal of Organic Chemistry