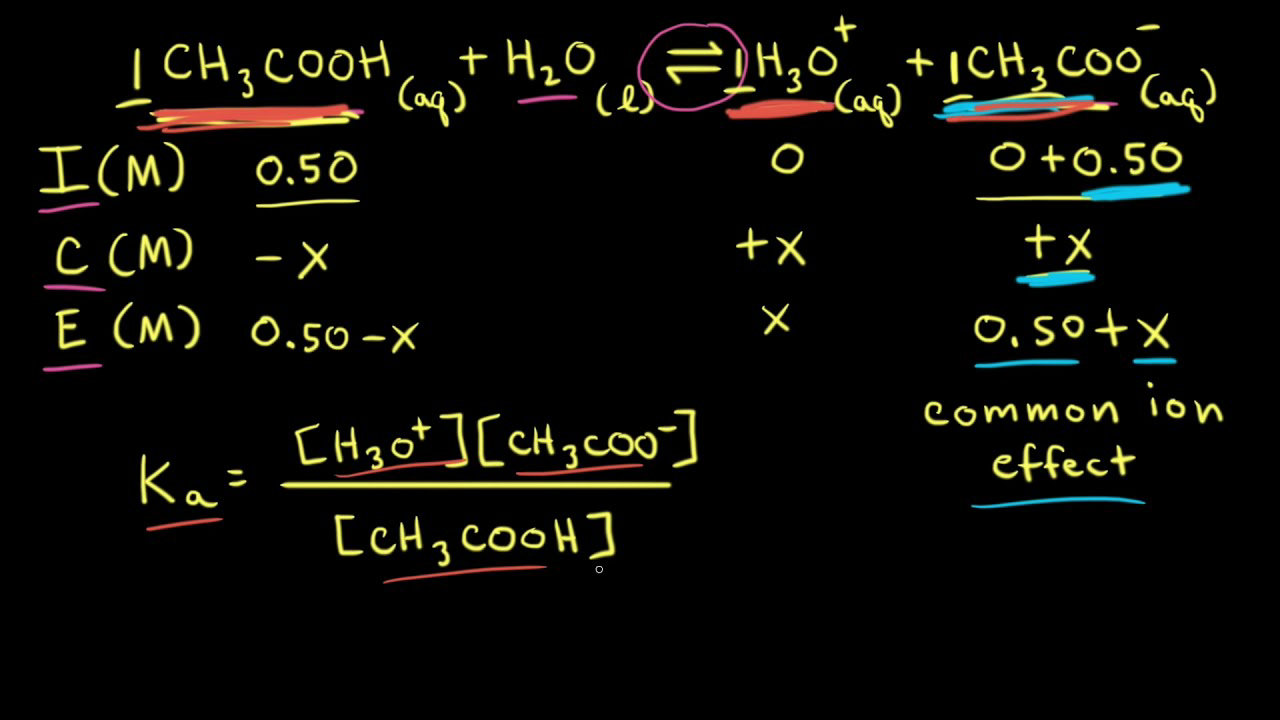
Worked example: Calculating the pH after a weak acid–strong base reaction (excess acid) (video) | Khan Academy

Types and Strengths of Acids and Bases in Ionic Equilibria | by Maverick Puah the Chemistry Guru | Medium
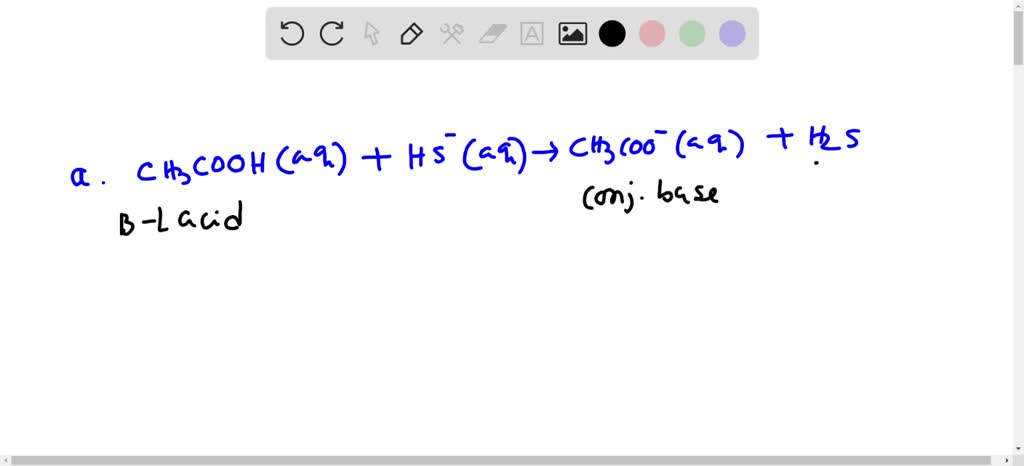
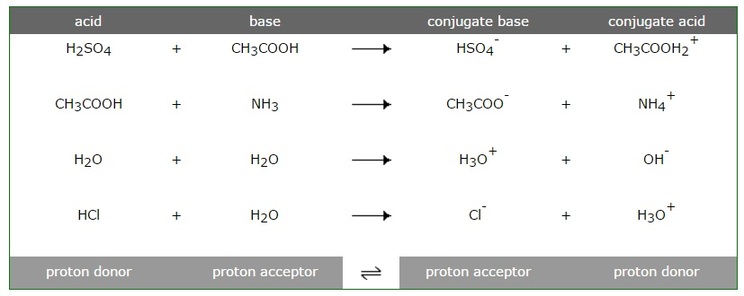
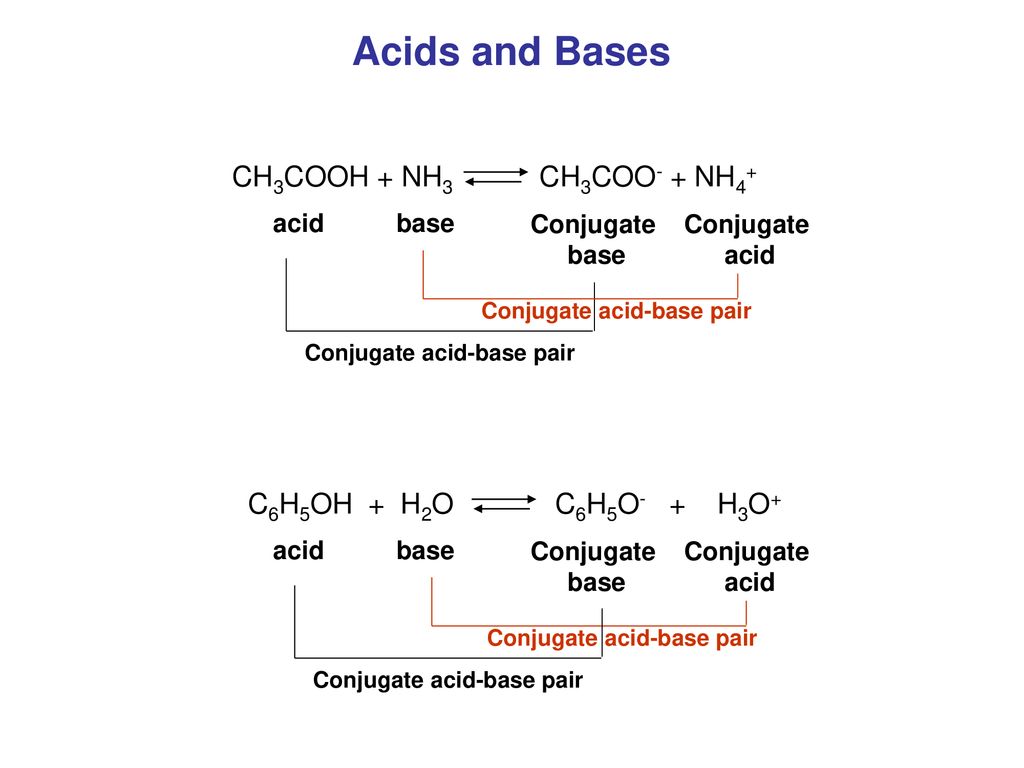
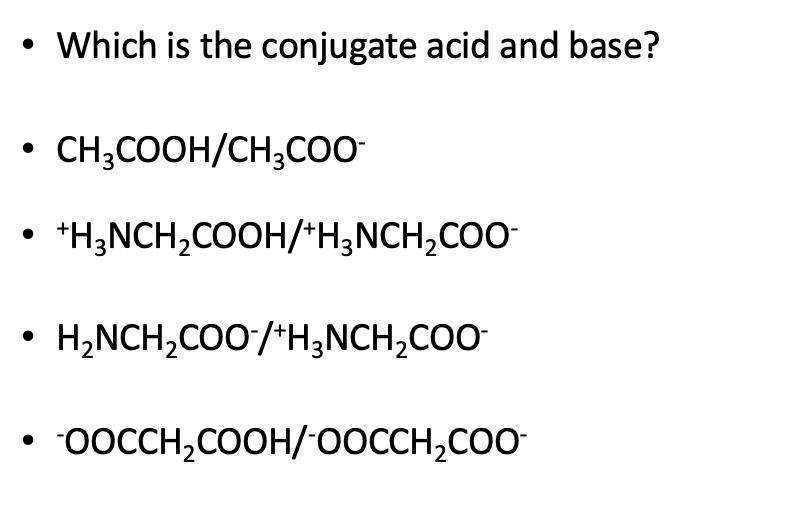
SOLVED: For each of the following reaction, identify the Bronsted-Lowry acids and bases and the conjugate acid-base pairs. a. CH3COOH(aq) + HS-(aq) —– CH3COO-(aq) + H2S(aq) b. F-(aq) + NH4+(aq) —– HF(aq) +

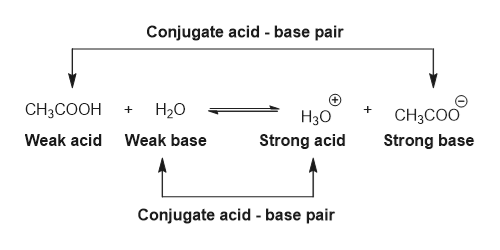
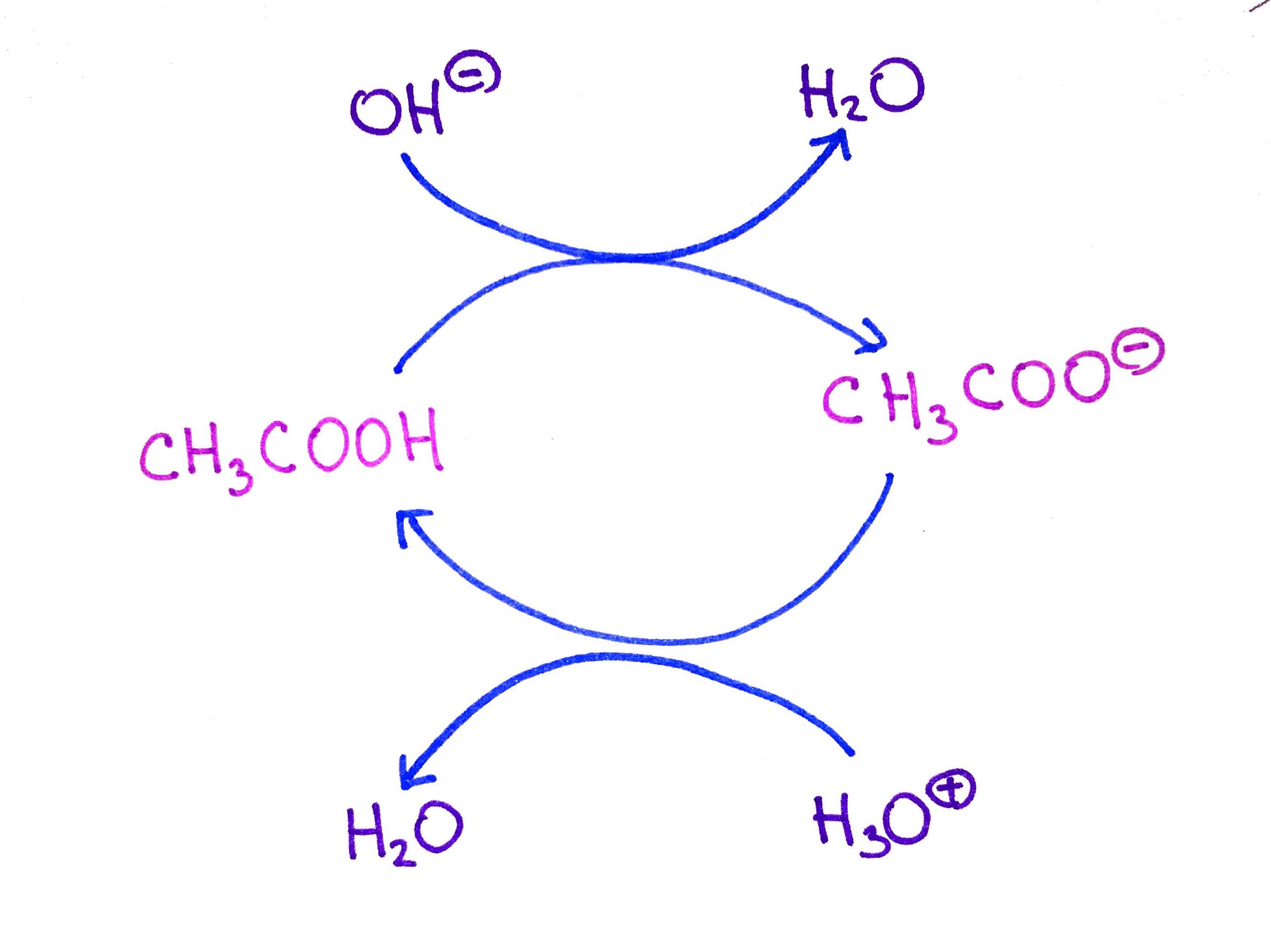
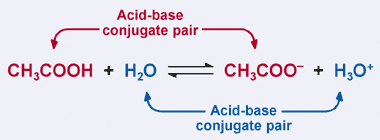
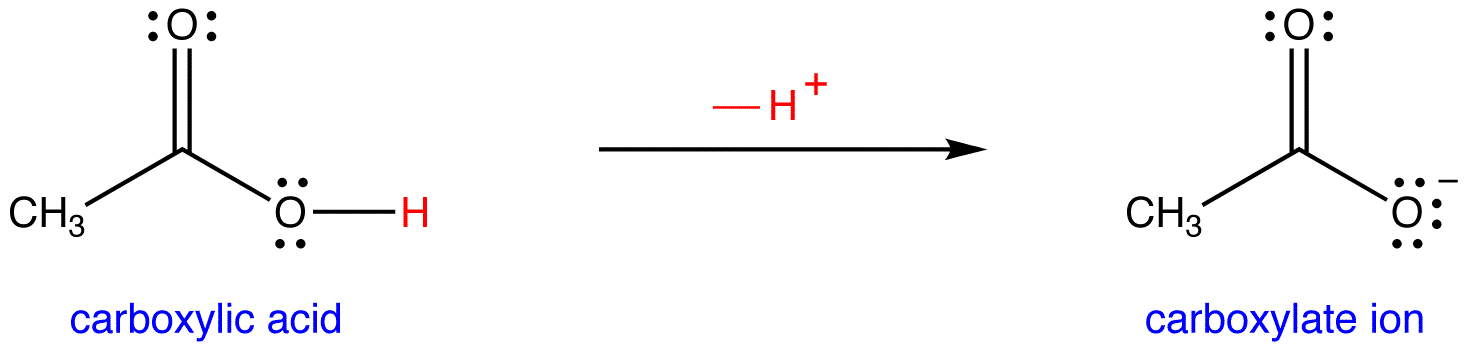
OneClass: 1. Acetic acid (CH3COOH) is a weak acid that reacts with water to form the acetate ion (CH ...