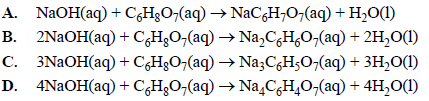organic chemistry - Role of hydrochloric acid and sodium hydroxide in isolation of citric acid from lemon juice - Chemistry Stack Exchange

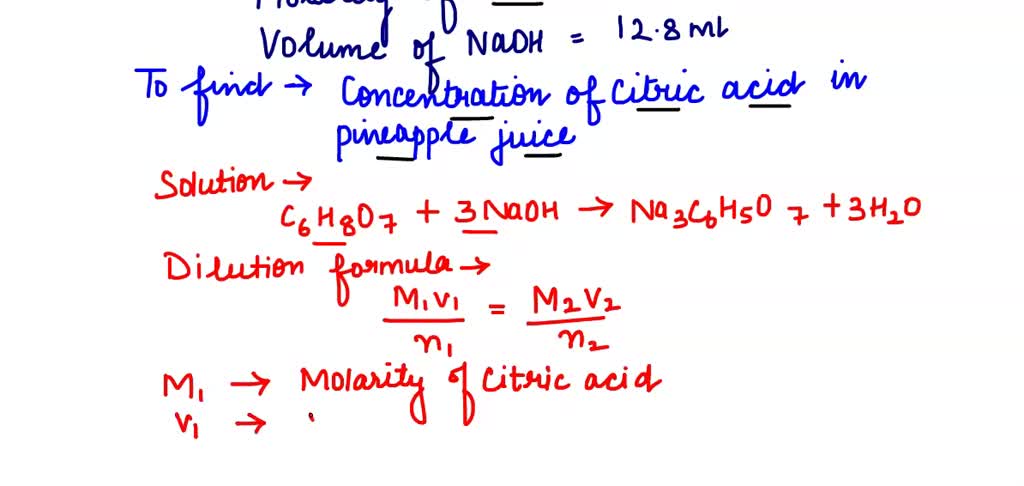
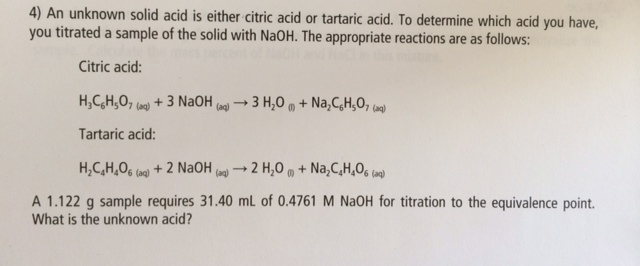
OneClass: Please show ALL work. Citric acid and sodium hydroxide react with each other according ...

Buffer Solution (citric acid, sodium hydroxide, hydrogen chloride) tracable to SRM from NIST and PTB pH 2.00 (25°C) Certipur® | Sigma-Aldrich
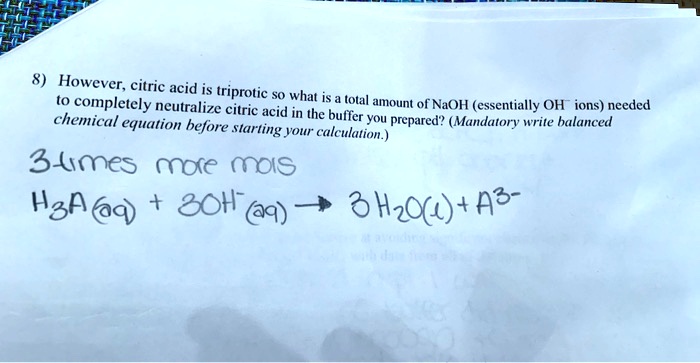
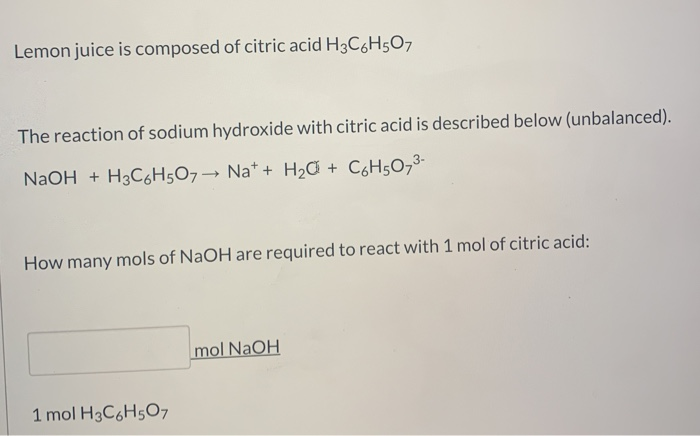
SOLVED: However, citric acid is triprotic completely neutralize SO what is total amount of NaOH (essentially OH ions) needed chemical equation before . citric acid in the buffer you prepared? (Mandatory write

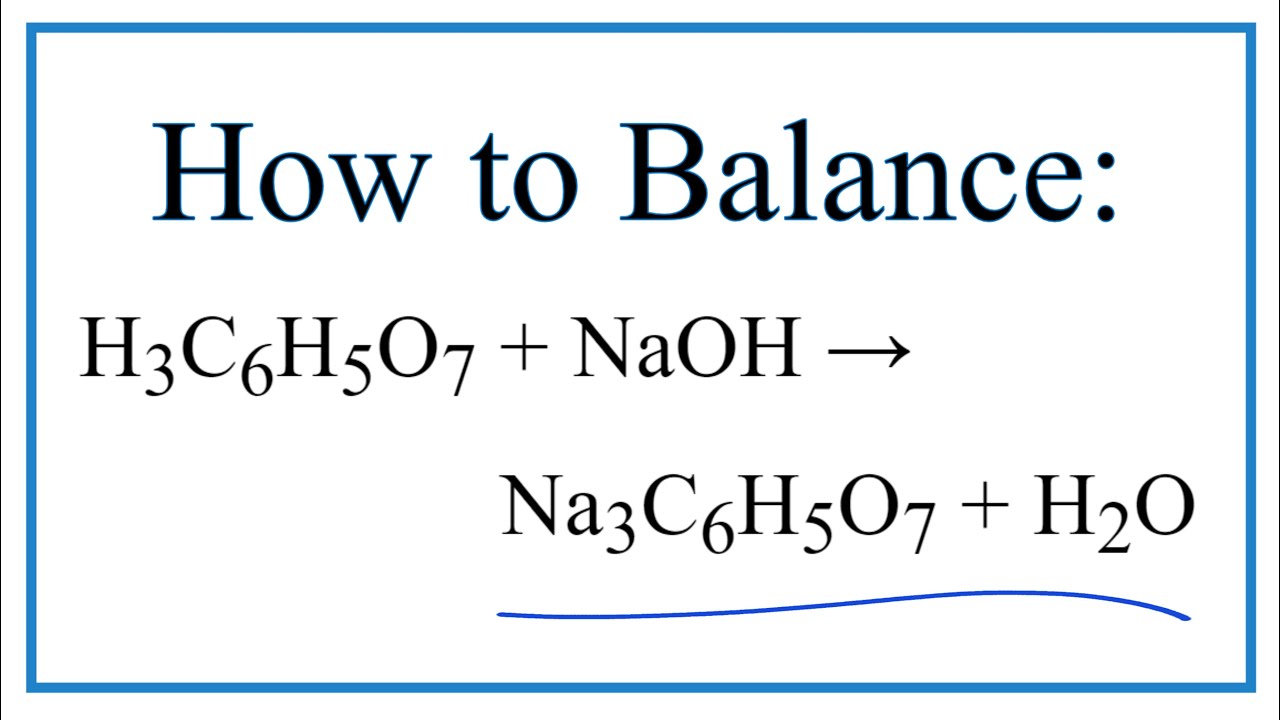
OneClass: White the net ionic equation for the reaction of citric acid and sodium hydroxide Chern21 L...