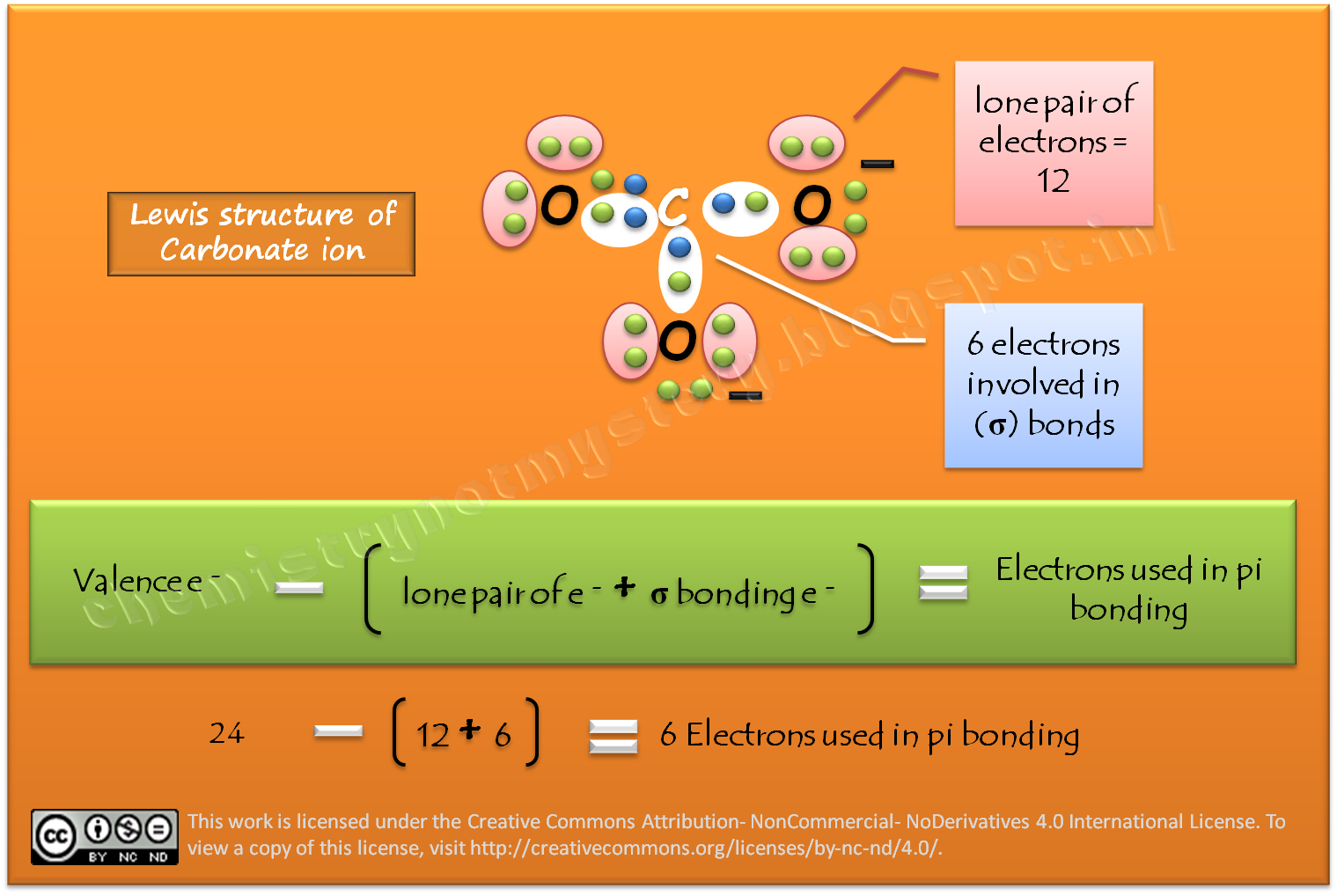
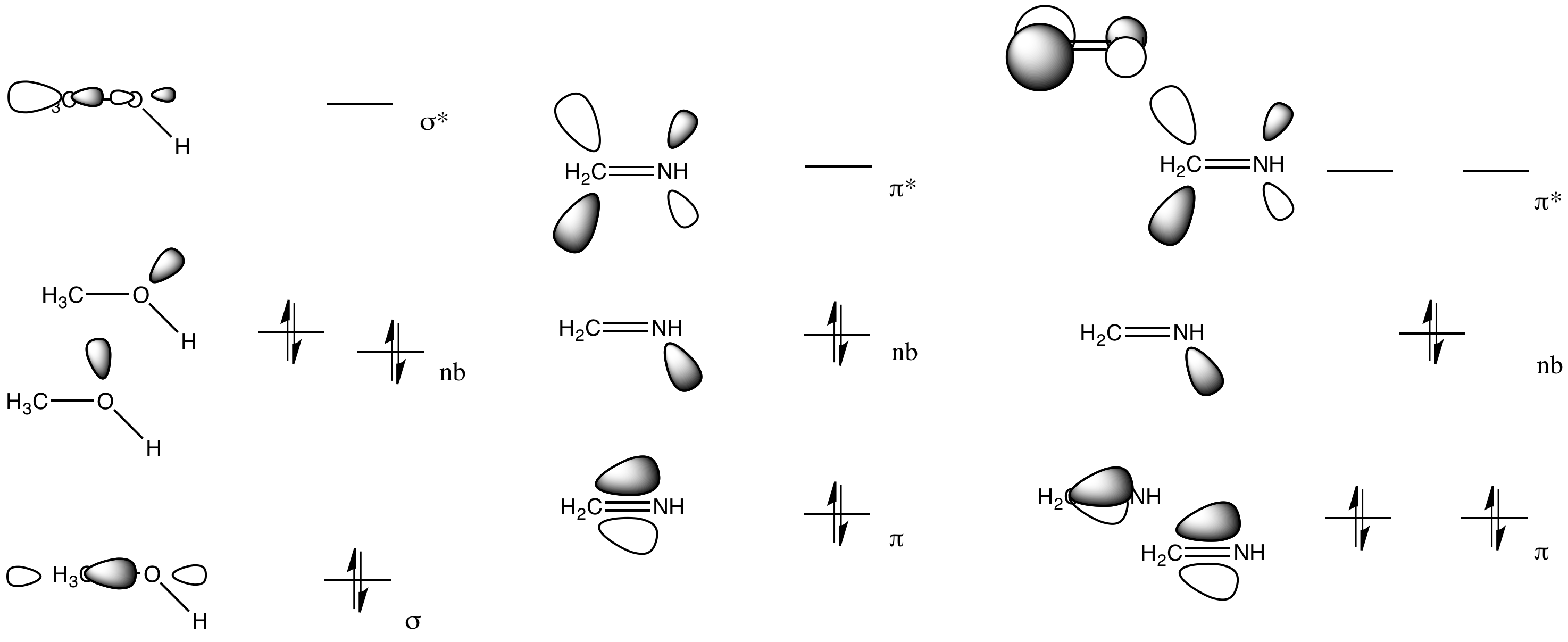
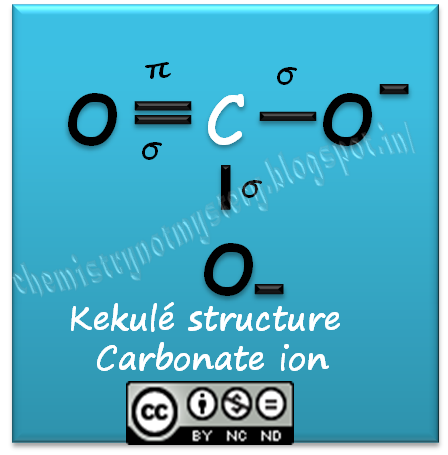
Describe the bonding in the CO32- ion using the LE model. How would the molecular orbital model describe the pi bonding in this species? | Homework.Study.com

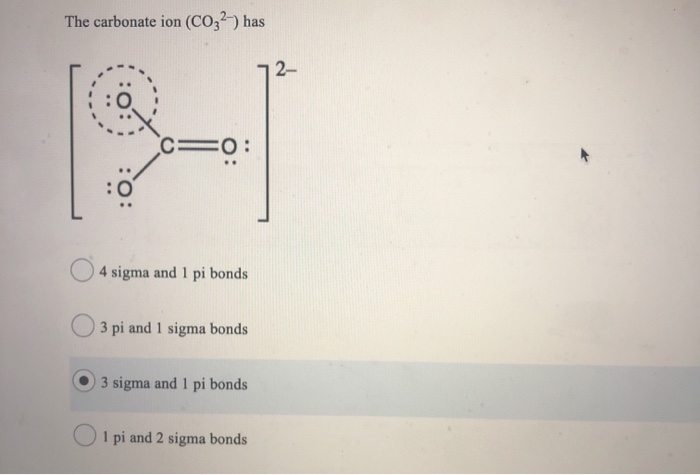
SOLVED: Select the number of sigma and pi bonds in the most favorable structure of the carbonate ion, CO32- one sigma bond, three pi bonds three sigma bonds; one pi bond three

Draw two equivalent resonance forms for bicarbonate ion, HCO3-. How many sigma bonds are there? How many pi bonds? | Homework.Study.com

Chemistry - Chemical Bonding (27 of 35) Lewis Structures - Resonance Structures - Carbonate Ion - YouTube

Draw an octet-rule Lewis structure for CO32-. State which orbitals or hybrids on C and O overlap to make each valence bond, state each bond type ( sigma or pi bond), and state

OneClass: Draw an octet-rule Lewis structure for CO32- State which orbitals or hybrids on C and O ove...