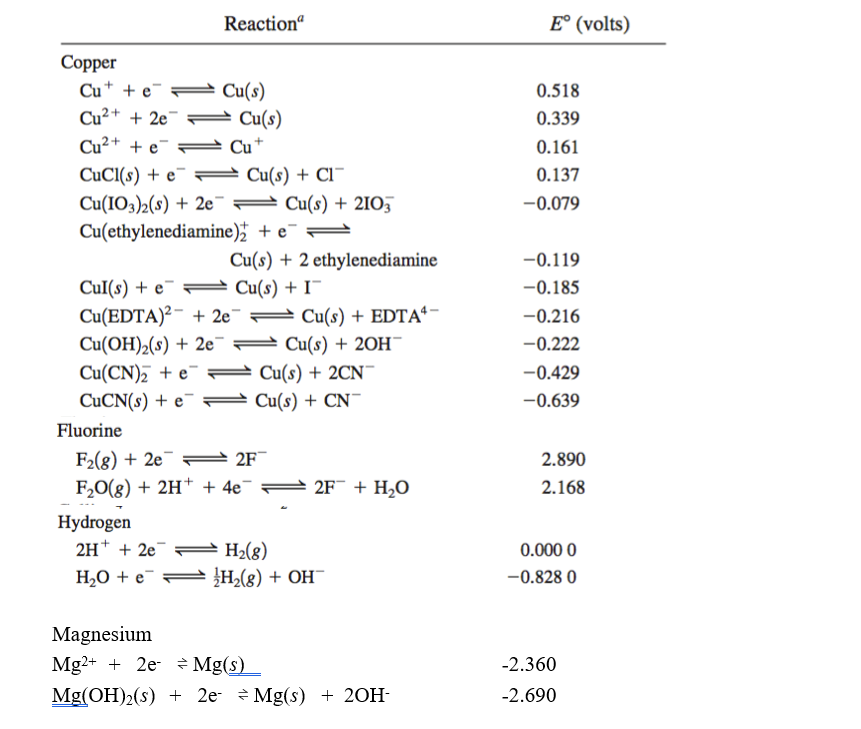
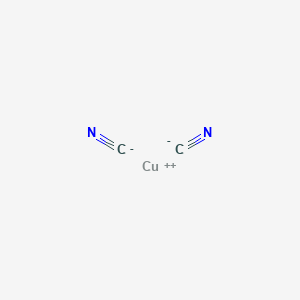
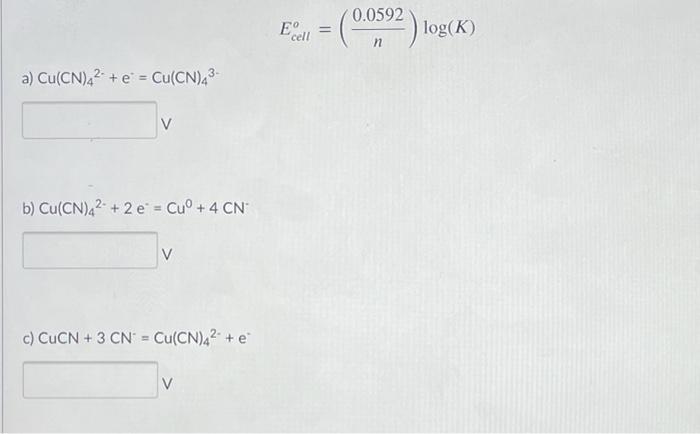
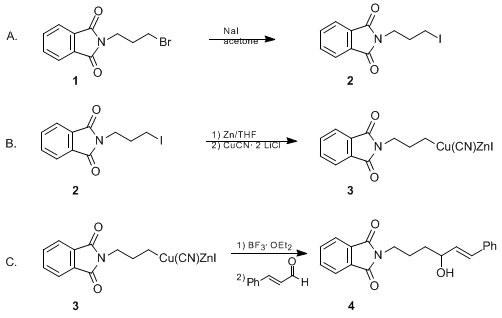
![CuSO4 decolourises on addition of KCN, the product formed isa)Cu2+ get reduced to form [Cu(CN)4]3–b)[Cu(CN)4]2–c)CuCNd)Cu(CN)2Correct answer is option 'A'. Can you explain this answer? | EduRev JEE Question CuSO4 decolourises on addition of KCN, the product formed isa)Cu2+ get reduced to form [Cu(CN)4]3–b)[Cu(CN)4]2–c)CuCNd)Cu(CN)2Correct answer is option 'A'. Can you explain this answer? | EduRev JEE Question](https://edurev.gumlet.io/ApplicationImages/Temp/3066490_07663d4f-9149-4179-9b55-c0b5cae5fd47_lg.png?w=360&dpr=2.6)
CuSO4 decolourises on addition of KCN, the product formed isa)Cu2+ get reduced to form [Cu(CN)4]3–b)[Cu(CN)4]2–c)CuCNd)Cu(CN)2Correct answer is option 'A'. Can you explain this answer? | EduRev JEE Question

Sequential colorimetric recognition of Cu2+ and CN− by asymmetric coumarin-conjugated naphthol groups in aqueous solution - ScienceDirect
Chemistry and structure by design: ordered CuNi(CN)4 sheets with copper(II) in a squareplanar environment

Molecular structure of copper-cyanide divalent specie (Cu(CN) 3 22 ),... | Download Scientific Diagram
![The cations and anions in Na[Cuen 2 ][Cu(CN) 4 ], with ellipsoids at... | Download Scientific Diagram The cations and anions in Na[Cuen 2 ][Cu(CN) 4 ], with ellipsoids at... | Download Scientific Diagram](https://www.researchgate.net/publication/241695146/figure/fig1/AS:601663278682115@1520459112307/The-cations-and-anions-in-NaCuen-2-CuCN-4-with-ellipsoids-at-the-50-level.png)
The cations and anions in Na[Cuen 2 ][Cu(CN) 4 ], with ellipsoids at... | Download Scientific Diagram

Reaction of Cu(CN)32− with H2O2 in water under alkaline conditions: Cyanide oxidation, Cu+/Cu2+ catalysis and H2O2 decomposition - ScienceDirect

Reaction of Cu(CN)32− with H2O2 in water under alkaline conditions: Cyanide oxidation, Cu+/Cu2+ catalysis and H2O2 decomposition - ScienceDirect