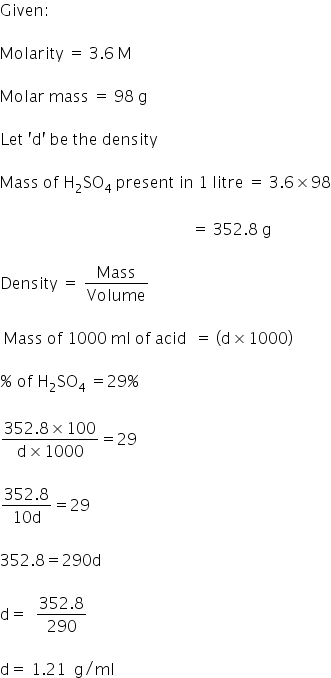Kinetics of selenium and tellurium removal with cuprous ion from copper sulfate-sulfuric acid solution | Semantic Scholar

The density (in g mL^-1 ) of a 3.60 M sulphuric acid solution H2SO4 (molar mass = 98 g mol^-1 ) will be:
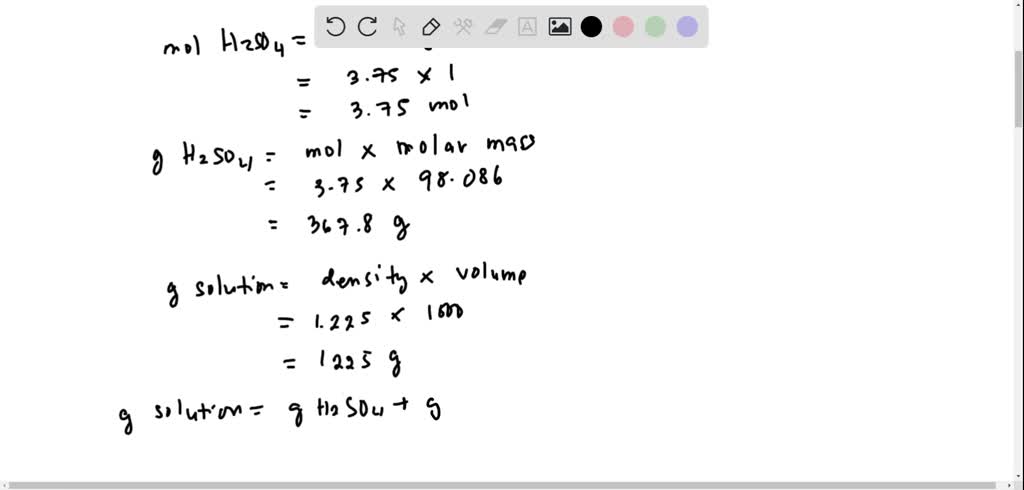
SOLVED:The density of a sulfuric acid solution taken from a car battery is 1.225 g / cm^3 . This corresponds to a 3.75 M solution. Express the concentration of this solution in

10mL of sulphuric acid solution (specific gravity= 1.84) contains 98% by weight of pure acid . - YouTube

Calculate the mass percent `(w//w)` of sulphuric acid in a solution prepared by dissovles 4 g of - YouTube

reactivity - How could mass increase when sulfuric acid is added to calcium carbonate? - Chemistry Stack Exchange

Chemical speciation effects on the volumetric properties of aqueous sulfuric acid solutions - ScienceDirect
The density (in g mL^–1 ) of a 3.60M sulphuric acid solution that is 29% H2SO4 (molar mass = 98g mol^–1 ) by mass will be. - Sarthaks eConnect | Largest Online Education Community