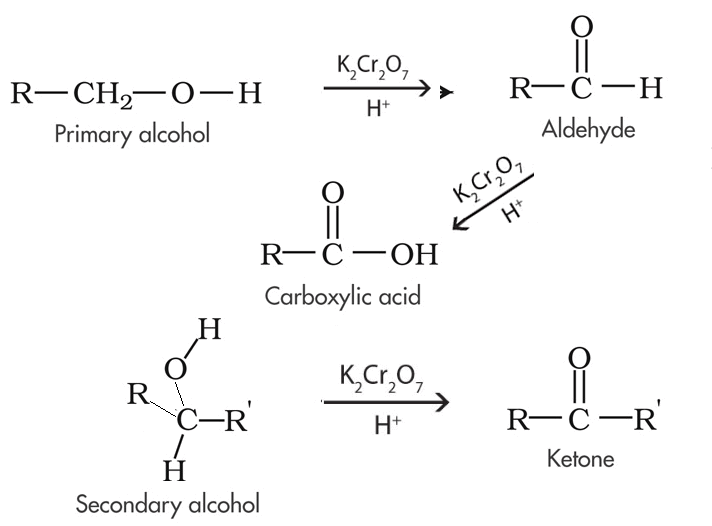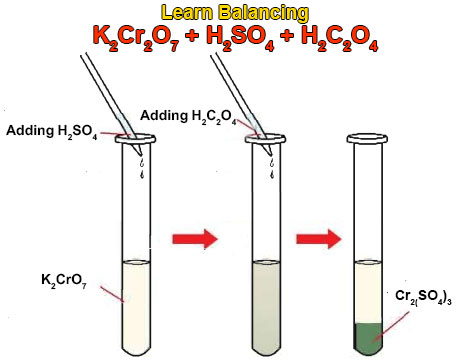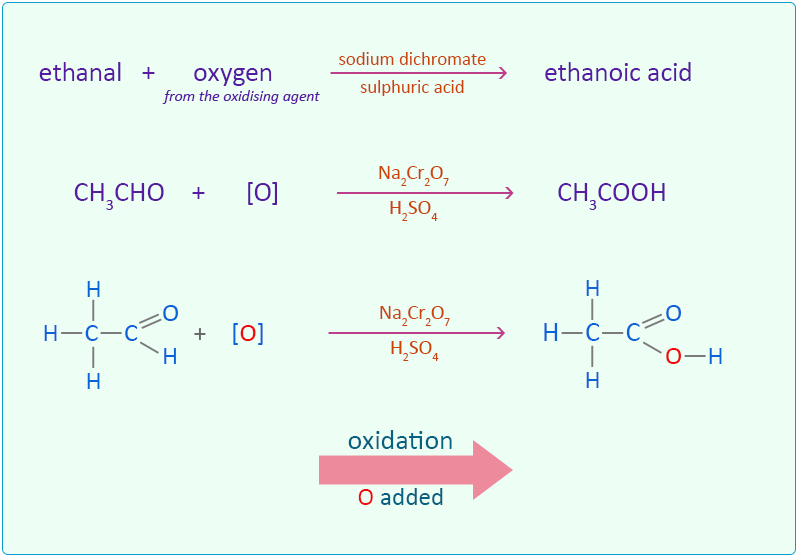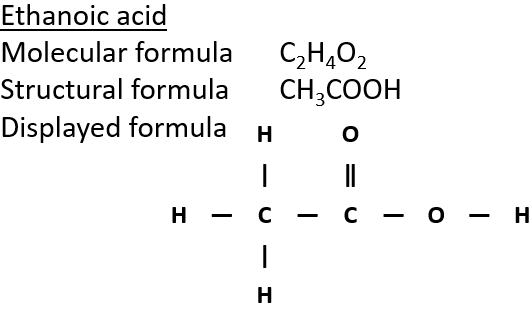
4:31 (Triple only) know that ethanol can be oxidised by: burning in air or oxygen (complete combustion), reaction with oxygen in the air to form ethanoic acid (microbial oxidation), heating with potassium

formula of = Potassium dichromate + Sulphuric acid = Potassium sulphate + Chromium sulphate + water + - Brainly.in

Wreite the balanced chemical equation of the following word equation.p Potassium dichromate + hydrochloric acid → potassium chloride + chromium chloride + water +chlorine

organic chemistry - Why is sulfuric acid used in the Jones oxidation of alcohols? - Chemistry Stack Exchange

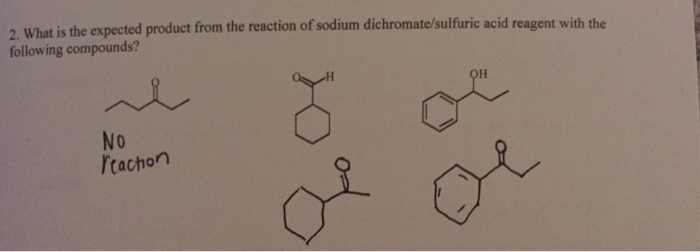
SOLVED: What are the major products in each of the following oxidation with sodium dichromate in sulfuric acid? OH Leeneannenen Mett# 4 Defete diennliit OH OH OH

organic chemistry - Reaction of naphthalene with sodium dichromate/sulfuric acid - Chemistry Stack Exchange

When a mixture of solid sodium chloride,potassium dichromate is heated with concentrated sulphuric acid orange red vapours are formed, then compound formed is :