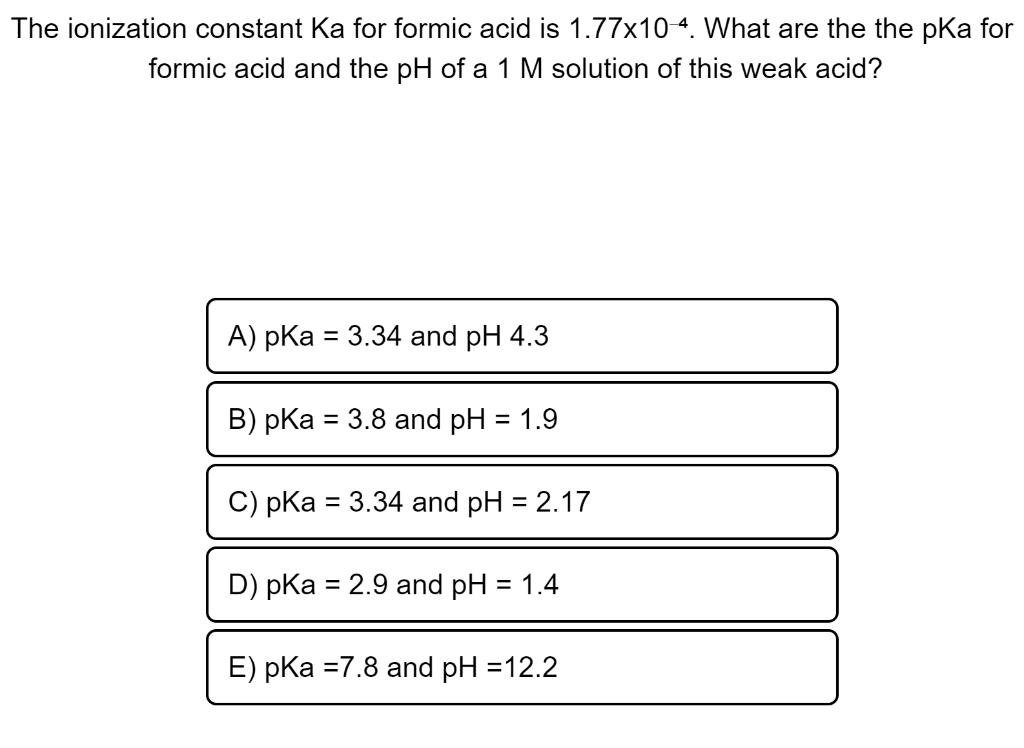
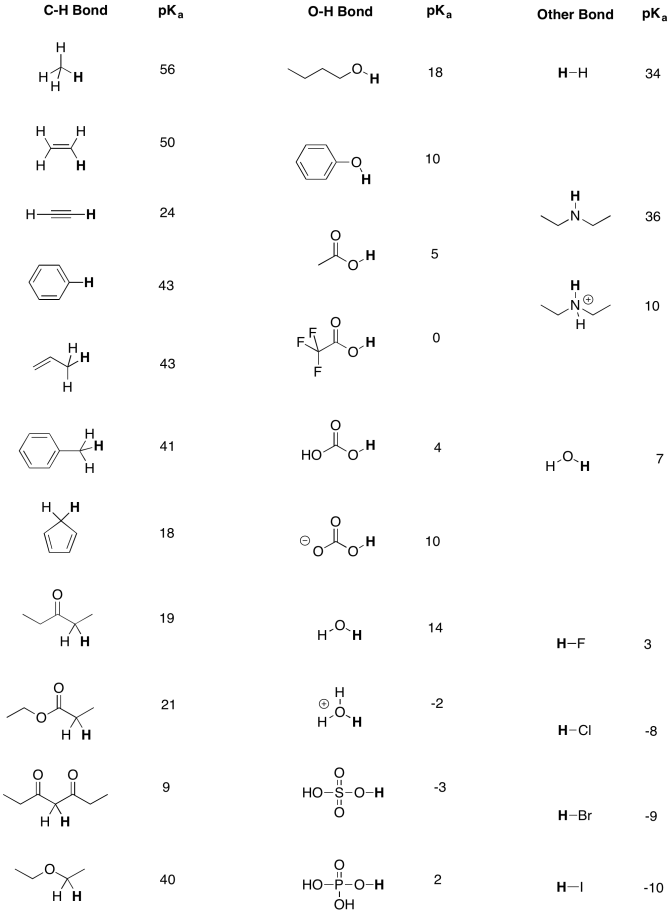
Value of dissociation constant of acetic acid is 10^-6 , where as dissociation constant of formic acid is 10^-5 . Which of the following will be the value of pKa (acetic acid) - pKa (formic acid)?

OneClass: What is the necessary ratio of potassium formate to formic acid to make a solution of pH = ...
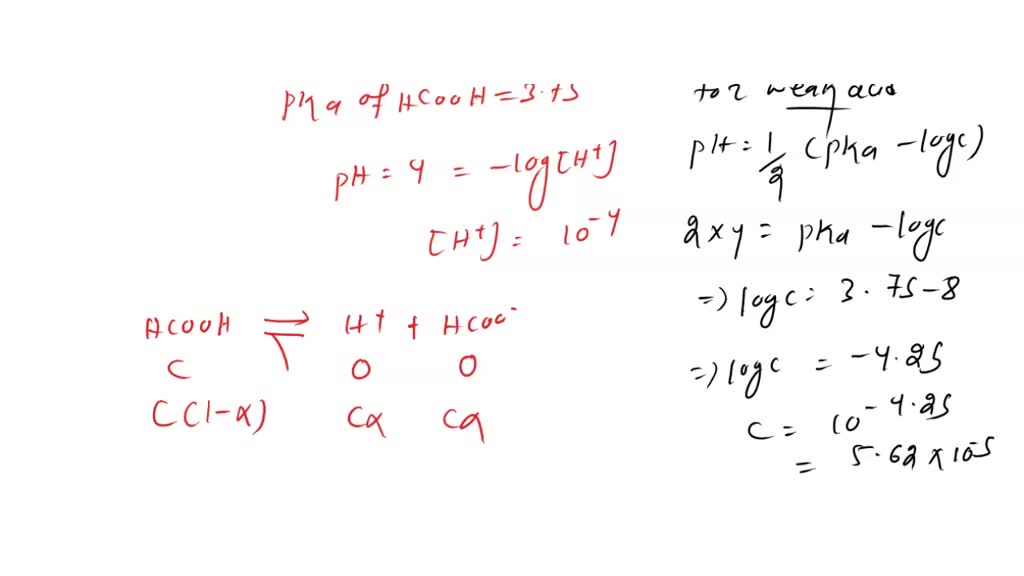
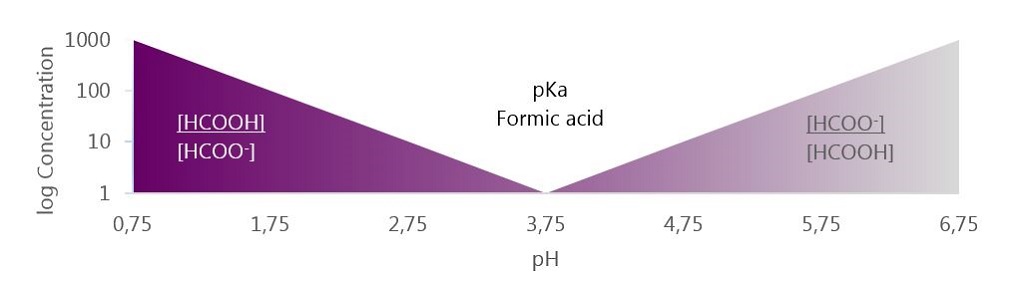
SOLVED: Formic acid (HCOOH) has a pKa of 3.75. (a) What percent of formic acid dissociates at pH 4? (b) What pH will the ratio of formic acid to formate be 2:1?

The comparison of pKa determination between carbonic acid and formic acid and its application to prediction of the hydration numbers - ScienceDirect

OneClass: The pKa of formic acid is 3.75. What is the buffering range (buffering region) of a buffer ...
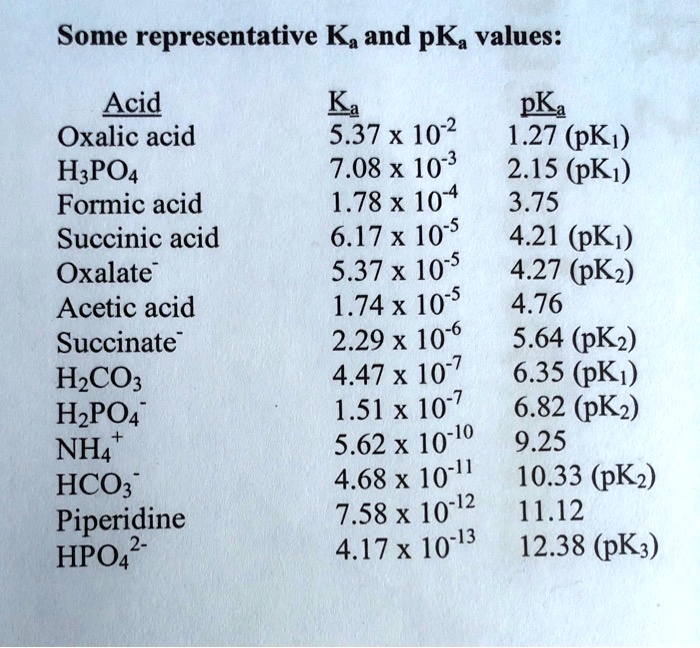
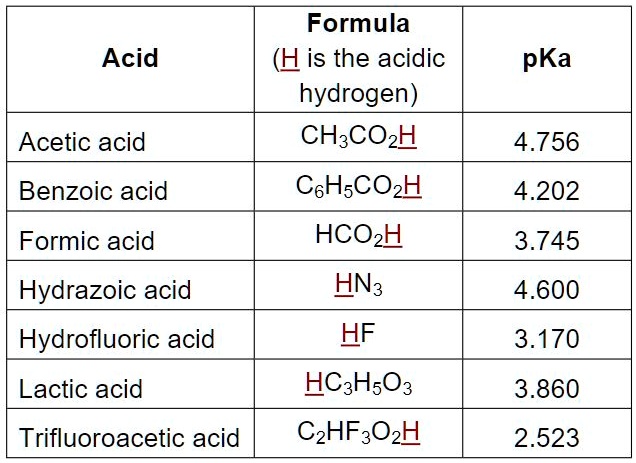
SOLVED: Some representative Ka and pKa values: Acid Oxalic acid HzPOa Formic acid Succinic acid Oxalate Acetic acid Succinate HzCOz HzPOA NH4' HCO; Piperidine HPOA2 Ka pKa 5.37 x 10-2 1.27 (pK,)

What is the pH of a 0.15 M solution of formic acid, HCOOH ? `{:("Formic Acid ",K_a),(HCOOH - YouTube
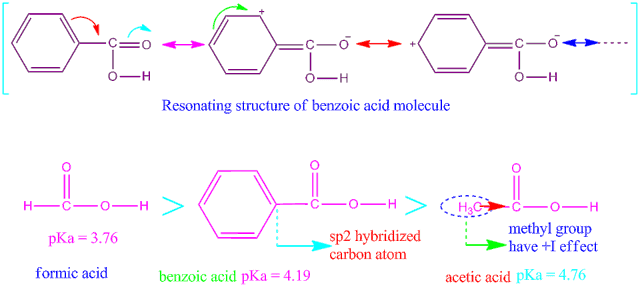
Benzoic acid-weak acid-stronger than acetic acid weaker than formic acid. | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium

If you have 500 mL of 0.15 M formic acid, what is the pH of this solution? What is the pKa? How many grams of sodium formate would you have to add



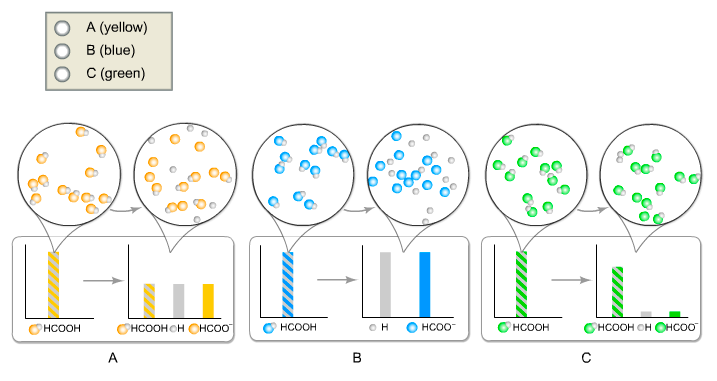

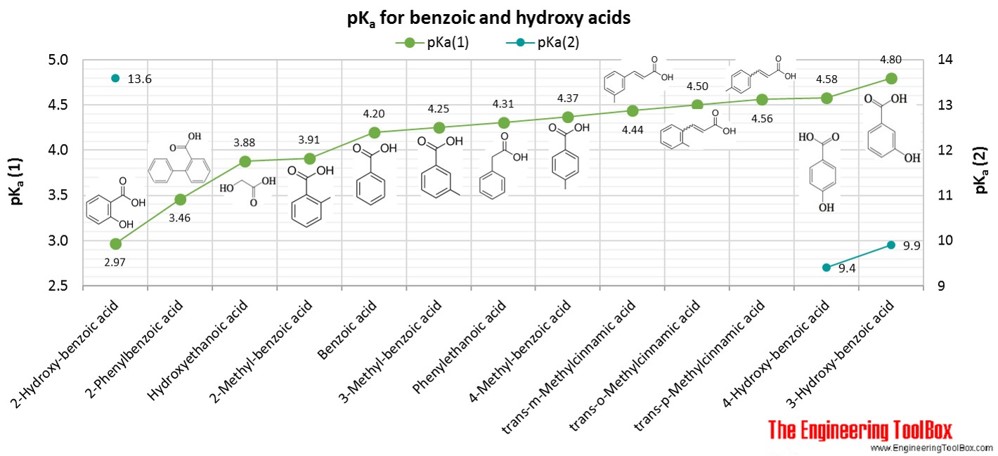
![Solved What is the ratio [A]/[HA] at pH 3.75? The pKa of | Chegg.com Solved What is the ratio [A]/[HA] at pH 3.75? The pKa of | Chegg.com](https://media.cheggcdn.com/media/5a3/5a32fe0d-21ca-4cf0-9a37-a59affbc86ba/phpMgWDCv.png)