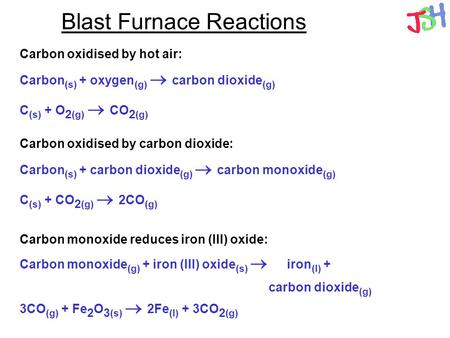
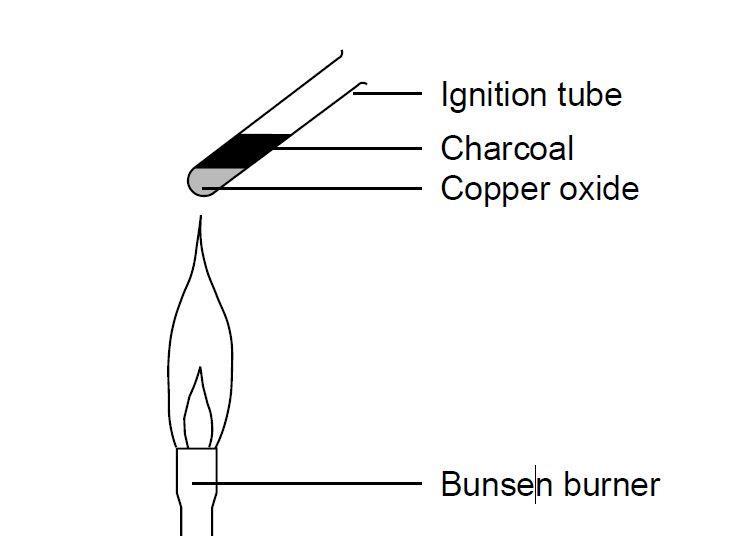
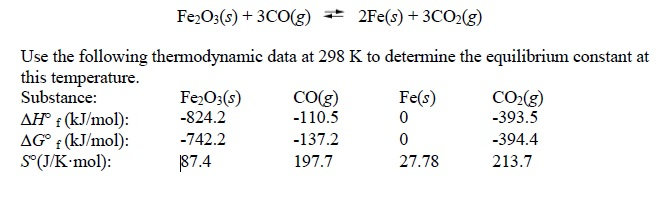
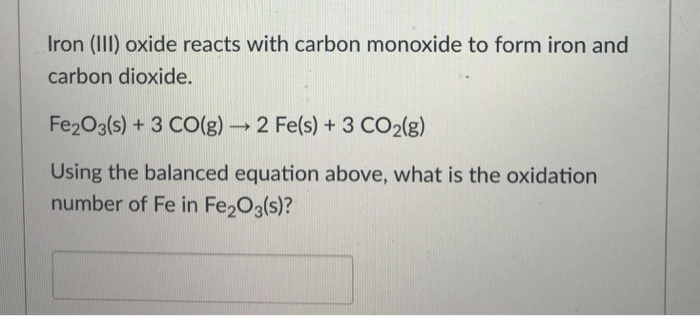Iron can be obtained by reduction of iron oxide (Fe3O4) with CO according to the reaction: Fe3O4 + 4CO → 3Fe + 4CO2 How many kg of Fe3O4 should be heated with

Iron can be obtained by reduction of iron oxide (Fe3O4) with CO according to the reaction: Fe3O4 + 4CO → 3Fe + 4CO2 How many kg of Fe3O4 should be heated with

Reduction of Carbon Dioxide by a Molybdenum-Containing Formate Dehydrogenase: A Kinetic and Mechanistic Study | Journal of the American Chemical Society

Iron(iii)oxide+Carbon monoxide=Iron+ Carbon dioxide Balanced Equation ||Fe2O3+CO=Fe+CO2 Balanced Equ. - YouTube

Reduction of Iron Oxides with Hydrogen—A Review - Spreitzer - 2019 - steel research international - Wiley Online Library

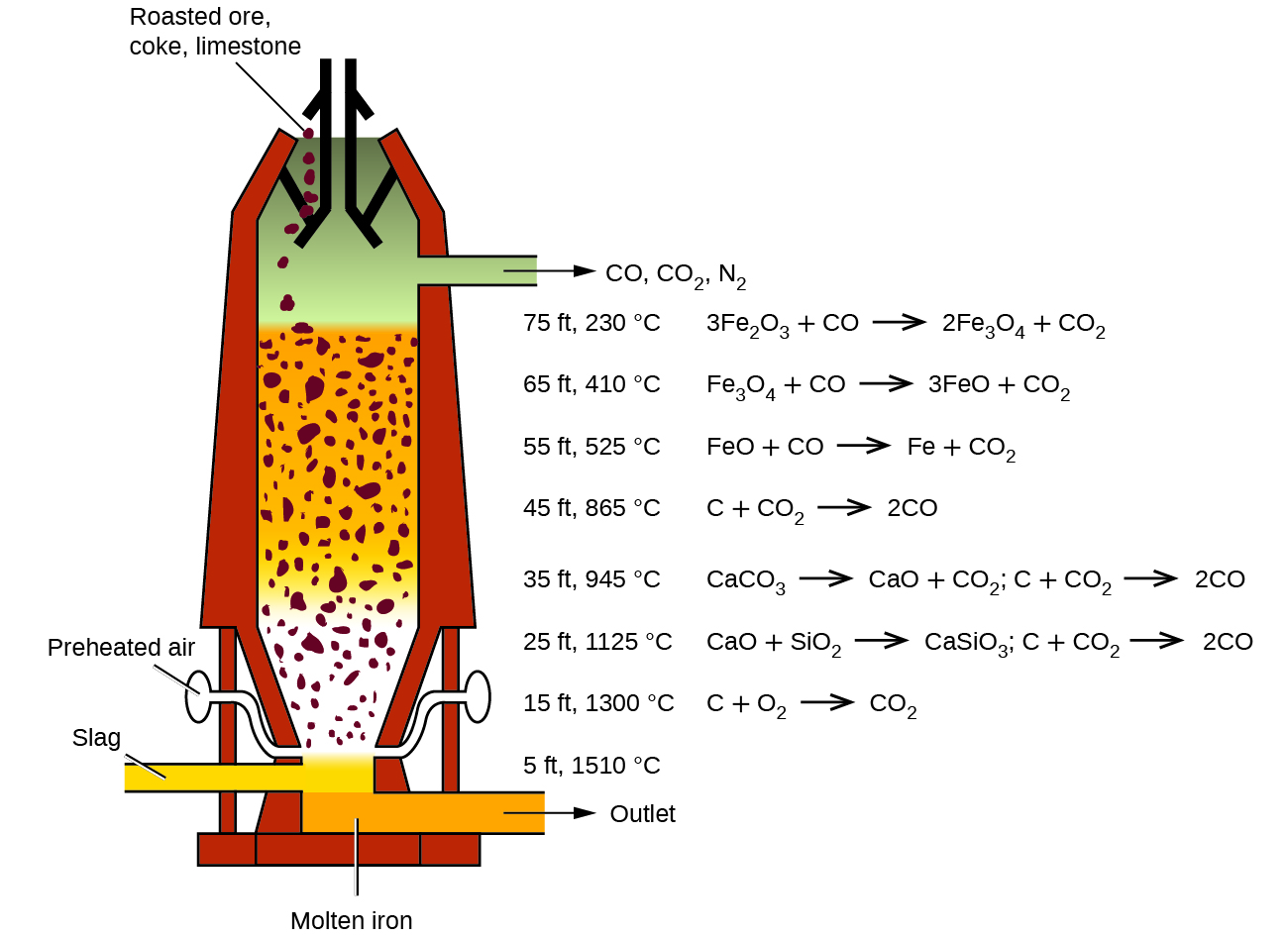
Question Video: Identifying the Compound That Reduces Iron Ore inside a Working Blast Furnace | Nagwa
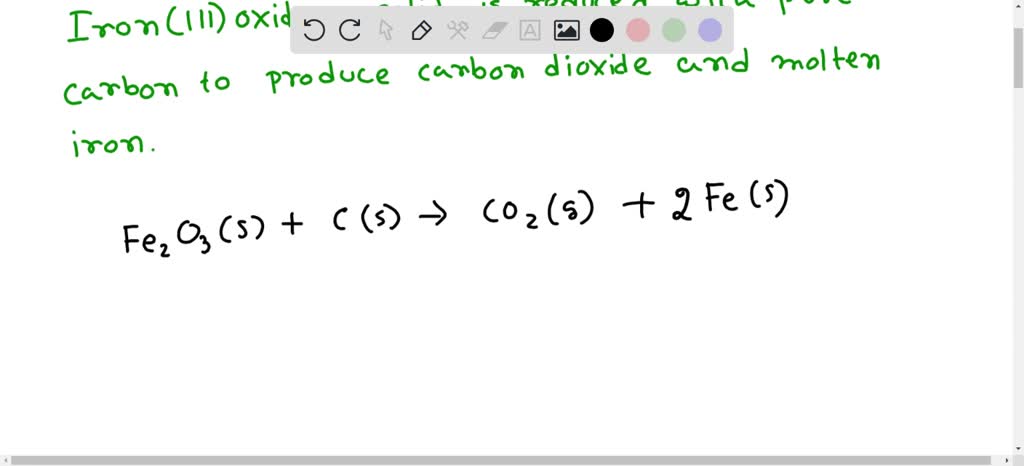
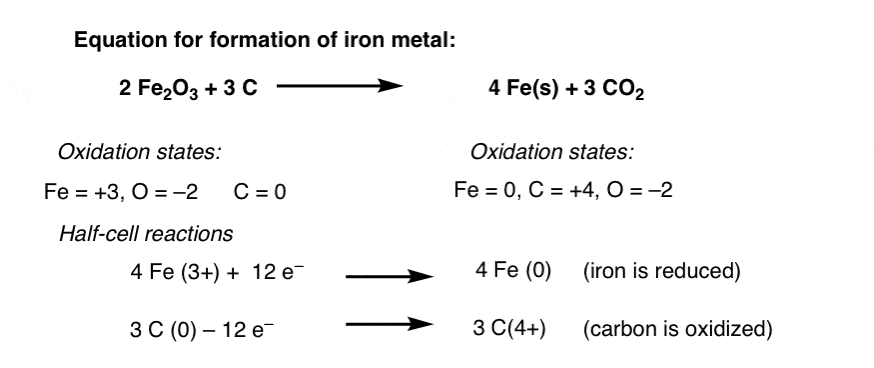
SOLVED: The balanced equation for the reaction occurring when iron(iii) oxide, a solid, is reduced with pure carbon to produce carbon dioxide and molten iron is

OneClass: Write a balanced equation for the reaction that occurs wher (a) iron(III) oxide is reduced ...

Causes chemistry of rusting rust prevention introduction to oxidation reduction REDOX reactions gcse igcse KS4 science chemistry revision notes revising
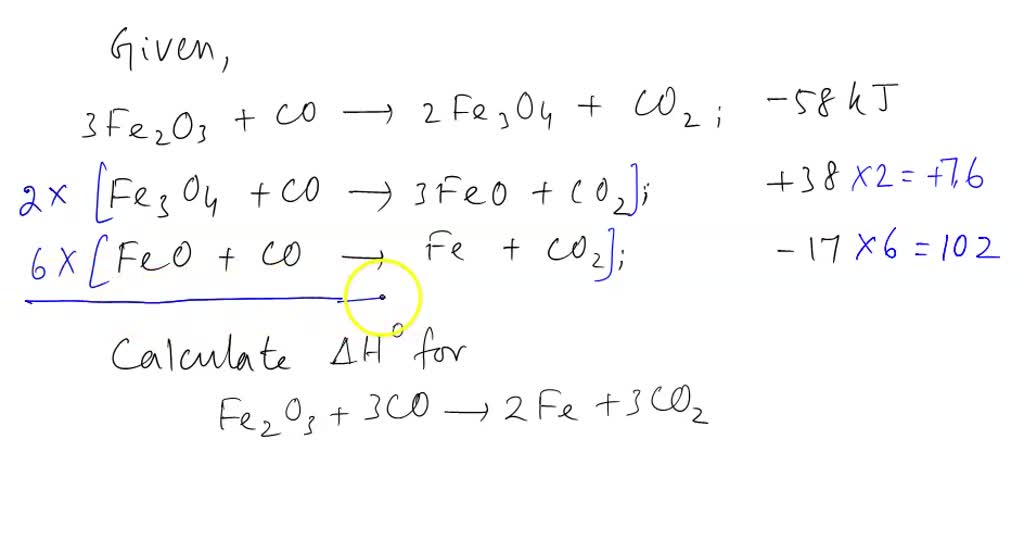
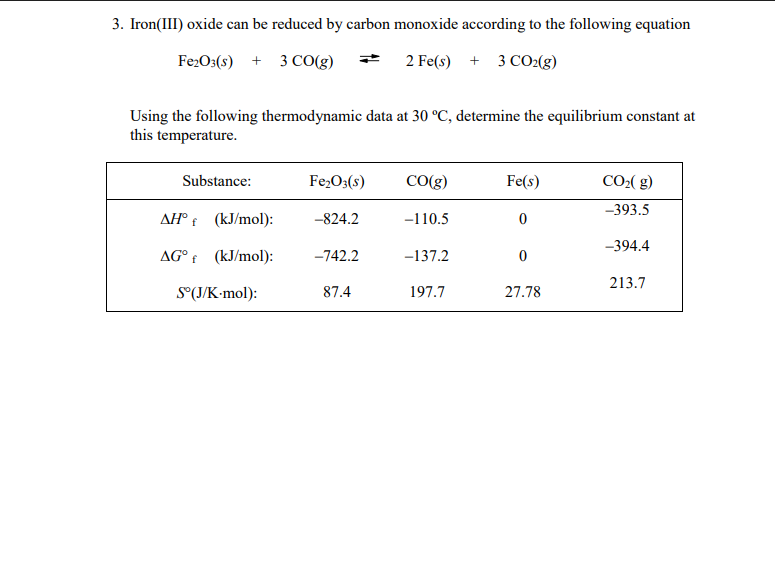
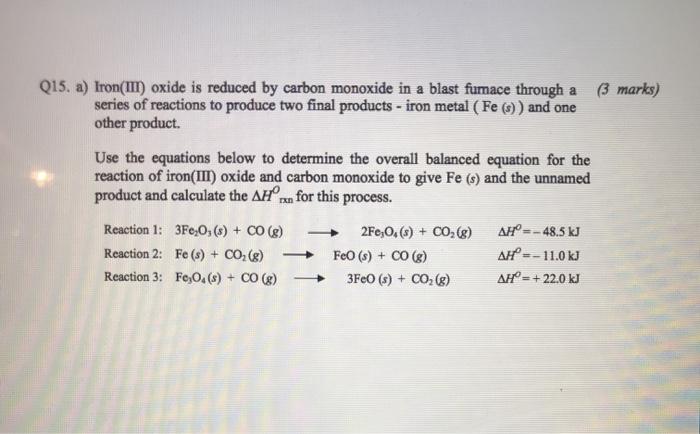
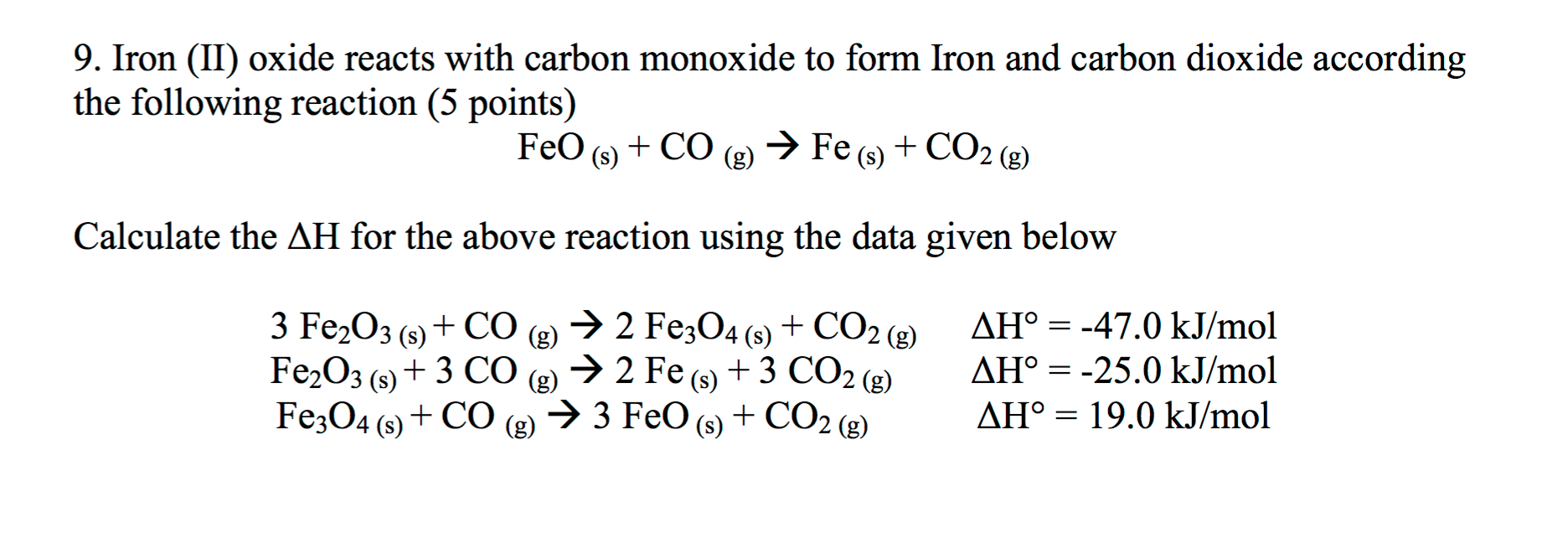
SOLVED: Part 1) Use the following equations to calculate the heat of the reaction for the reduction of iron oxide to iron with carbon monoxide. 3Fe2O3(s) + CO(g) → 2Fe3O4(s) + CO2(g)

Reduction of Iron Oxides with Hydrogen—A Review - Spreitzer - 2019 - steel research international - Wiley Online Library
Iron(III) oxide is reduced by carbon to give iron and carbon monoxide. What is the purity of the iron(III) oxide if only 55.6g of ion is obtained during the reduction? - Quora