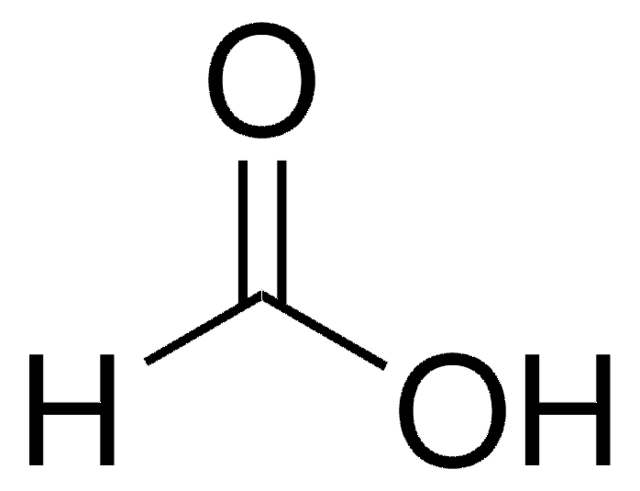
Formic Acid as Carbon Monoxide Source in the Palladium-Catalyzed N-Heterocyclization of o-Nitrostyrenes to Indoles | The Journal of Organic Chemistry

Energies | Free Full-Text | Cost Efficiency Analysis of H2 Production from Formic Acid by Molecular Catalysts

SOLVED: The Ka for formic acid, HCOOH, is 1.77 * 10^-4. HCOOH (aq) + H2O (l) ⇌ HCOO- (aq) + H3O+ (aq) What is the pH of a buffer made from 2.0

What is the pH of a 0.15 M solution of formic acid, HCOOH ? `{:("Formic Acid ",K_a),(HCOOH - YouTube

SOLVED: The value of Ka for formic acid HCOOH is 1.80x10^-4. Write the equation for the reaction that goes with this equilibrium constant: (Use H2O instead of H+.)

Reaction and separation system for CO2 hydrogenation to formic acid catalyzed by iridium immobilized on solid phosphines under base-free condition - ScienceDirect

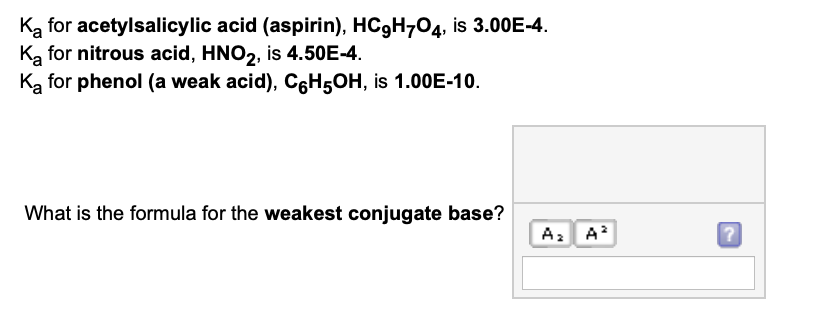
The Ka values of formic acid and acetic acid are respectively 1.77 × 10^-4 and 1.75 × 10^-5 . The ratio of the acid strength of 0.1M acid is:

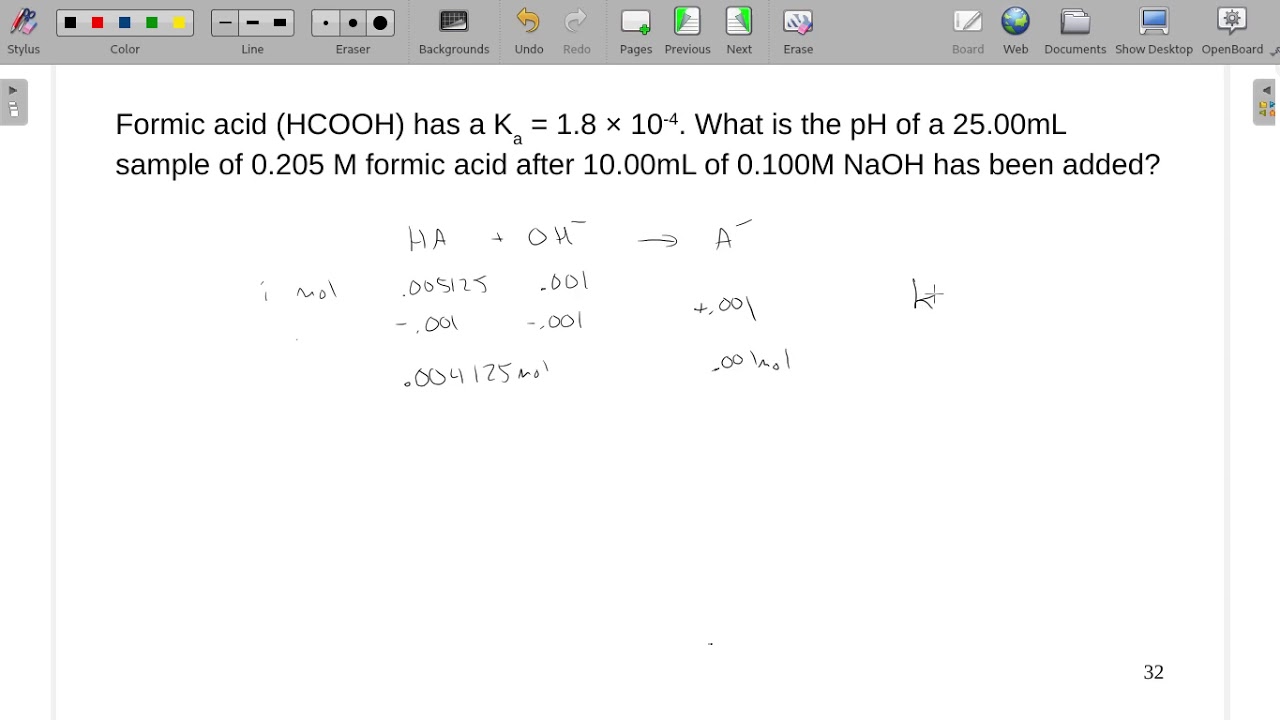
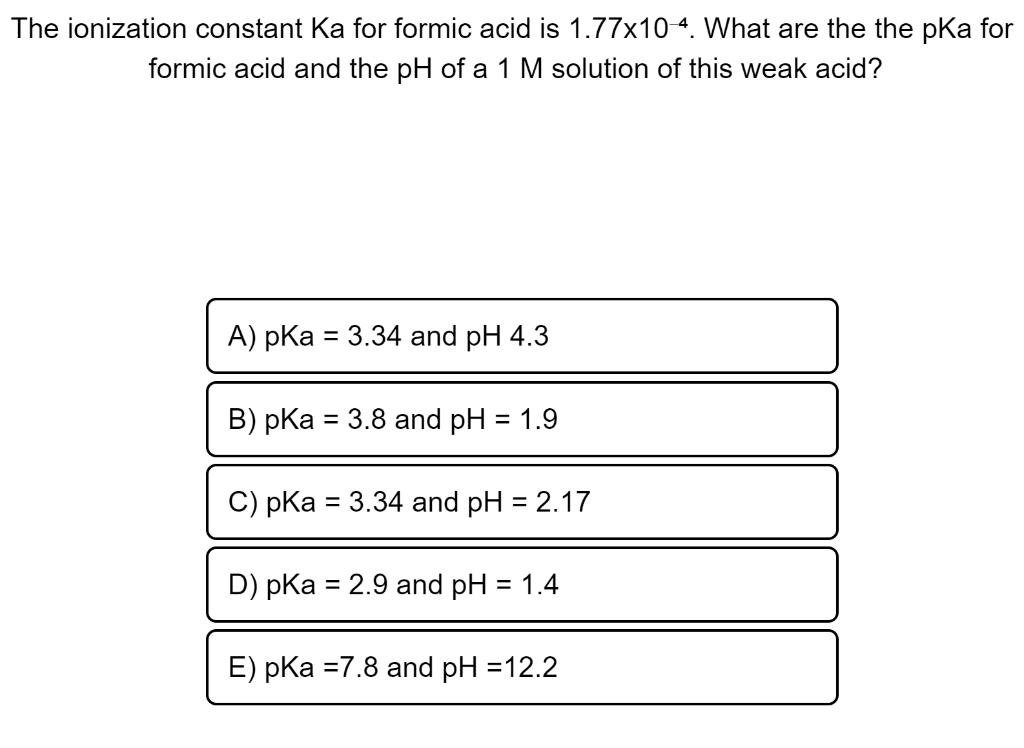
When a solution of formic acid was titrated with KOH solution, the pH of the solution was 3.65 when half the acid was neutralized. Calculate Ka(HCOOH) .
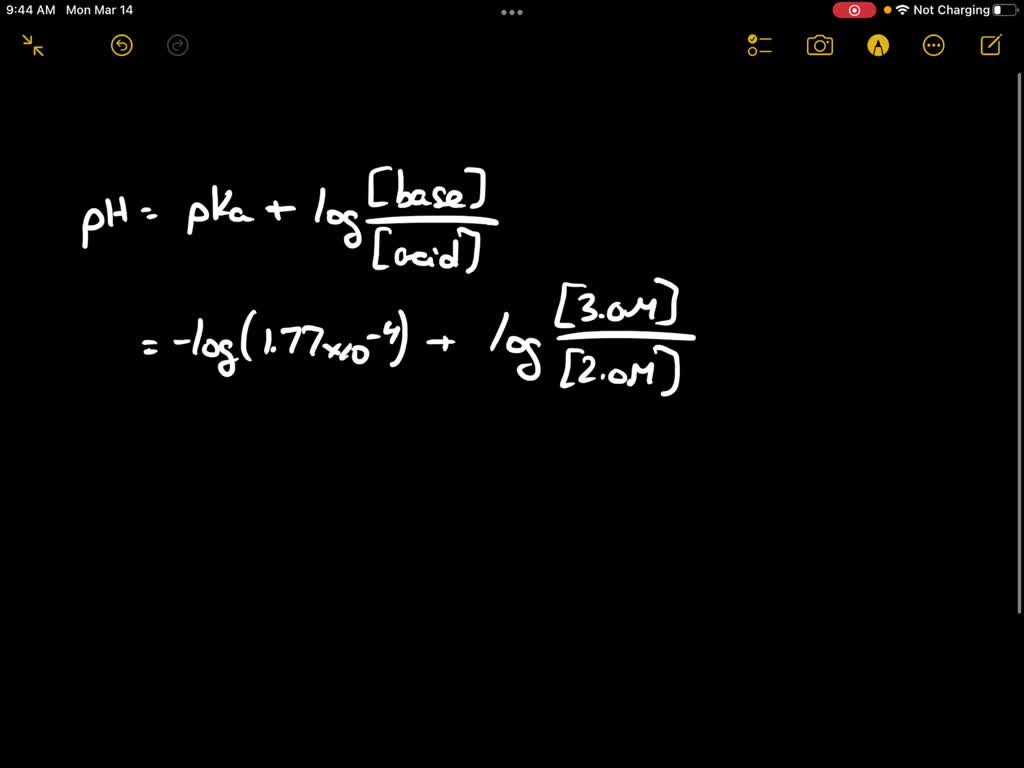
SOLVED: The Ka for formic acid, HCOOH, is 1.77 * 10^-4. HCOOH (aq) + H2O (l) ⇌ HCOO- (aq) + H3O+ (aq) What is the pH of a buffer made from 2.0
Formic acid, 50 ml, glass, 50 ml, CAS No. 64-18-6 | Starting material for eluent mixtures | Eluent additives for LC-MS | LC-MS | Liquid chromatography (LC, HPLC, LC-MS) | Chromatography | Applications | Carl Roth - International




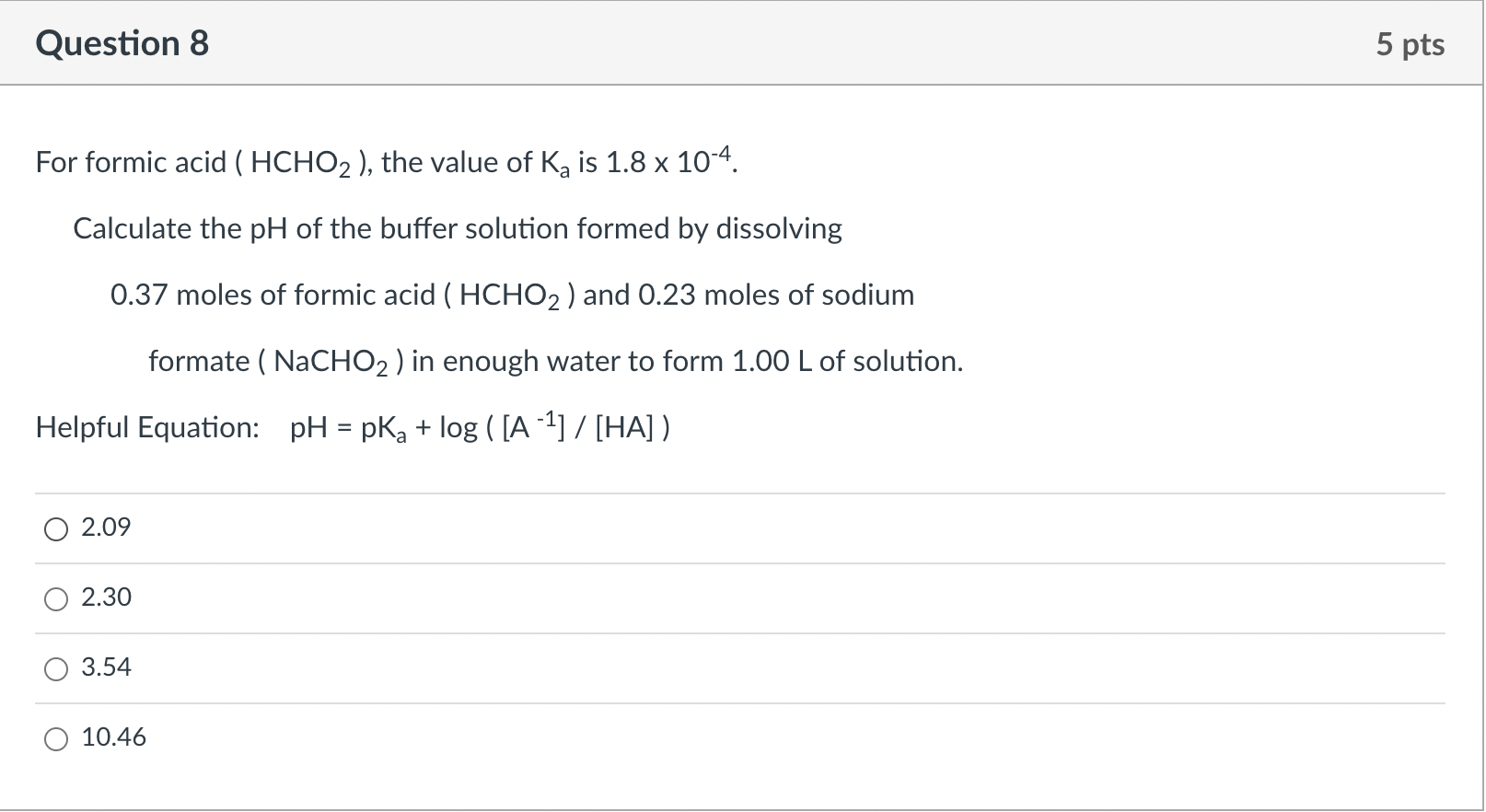



![a. A 0.1M solution of Formic acid [HCOOH] has Ka=1.77×10−4. Calculate (i).. a. A 0.1M solution of Formic acid [HCOOH] has Ka=1.77×10−4. Calculate (i)..](https://storage.googleapis.com/filo-classroom-notes/thumb_classroom_27777169_812PT.jpeg)