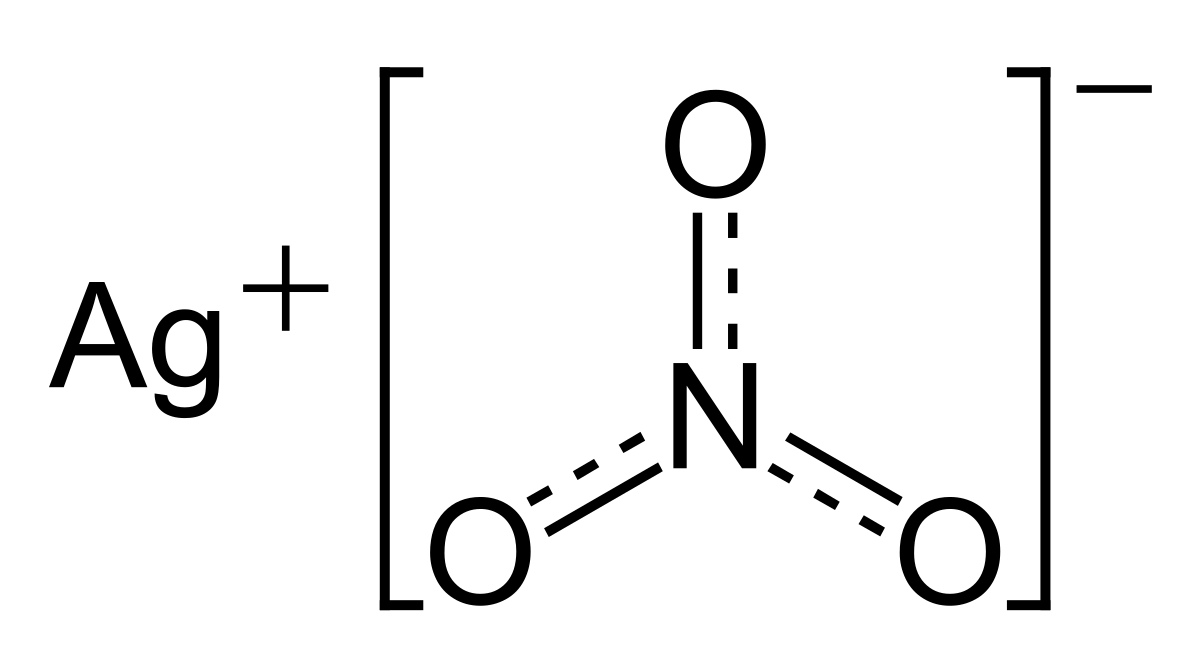
Direct transformation of AgNO3 complex encapsulated Fullerene (C60) microcrystal on solid silver Nitrate Crystal without organic Ligands - Hou - 2020 - Applied Organometallic Chemistry - Wiley Online Library
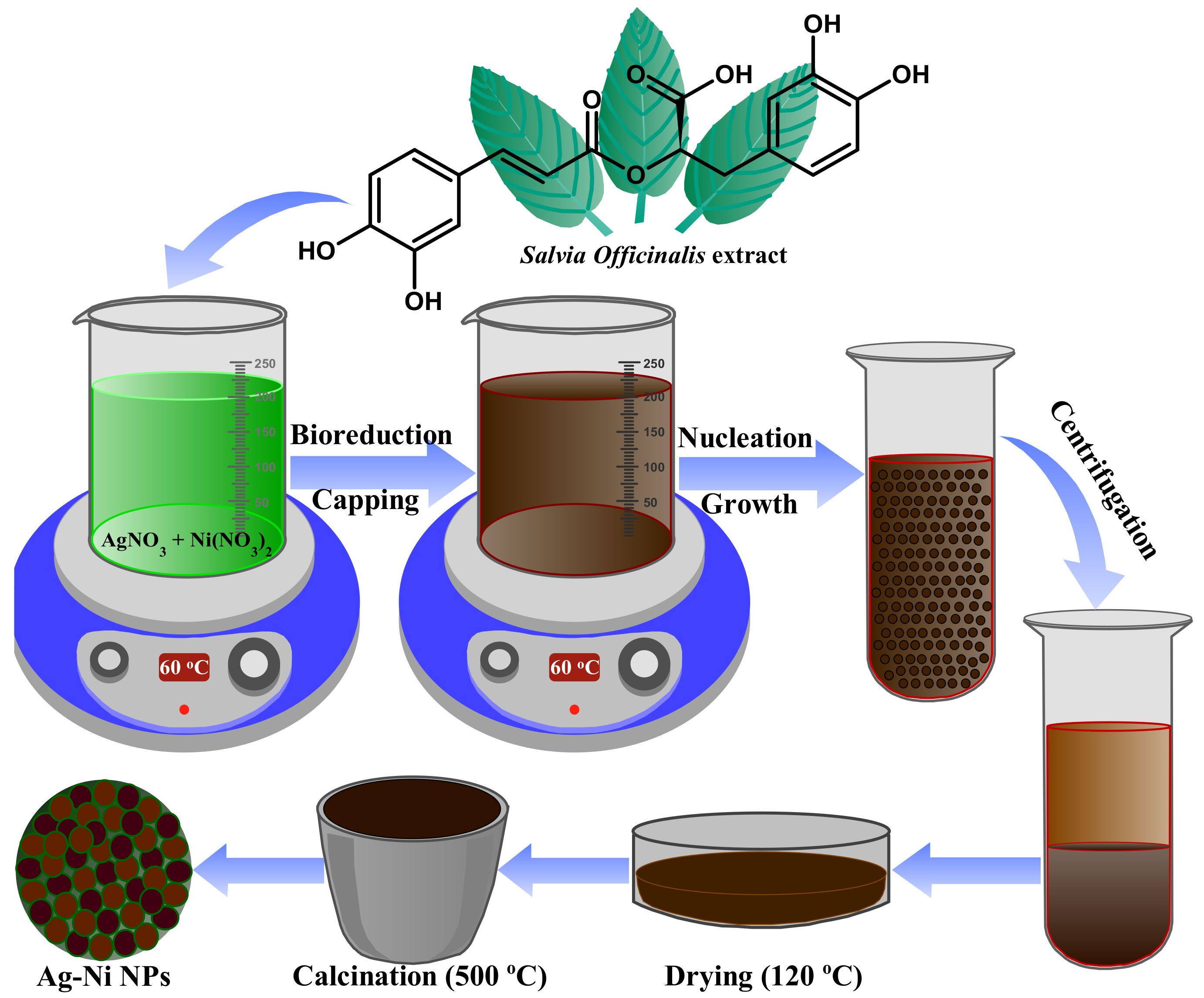
JoF | Free Full-Text | Combination Effect of Novel Bimetallic Ag-Ni Nanoparticles with Fluconazole against Candida albicans
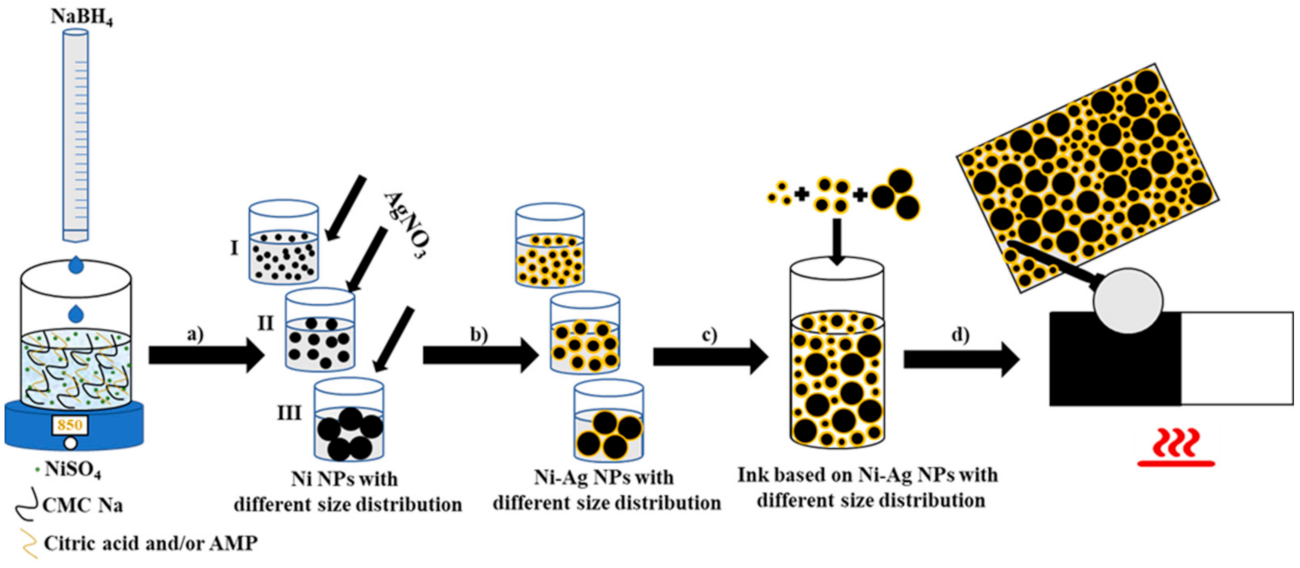
Materials | Free Full-Text | Polydispersity vs. Monodispersity. How the Properties of Ni-Ag Core-Shell Nanoparticles Affect the Conductivity of Ink Coatings
69 Hydrocarbon (A), C6H10 on treatment with H2/Ni, H2/Lindlar catalyst and Na/liquid NH3 forms three different reduction products (B), (C) and (D) respectively. (A) does not form any salt with ammoniacal AgNO3

Dynamic evolution and reversibility of single-atom Ni(II) active site in 1T-MoS2 electrocatalysts for hydrogen evolution | Nature Communications

SOLVED: Select the correct balanced net ionic equation for each of the following reactions occuring in aquequssolution Cu(NO3h2 Ni(NO3)2 AgNO3 NaCl Agcl NaNO3 Ca(NO3h2 KzCo3 Caco3 2KNO3 Cuz+ 2NO3 Ni(NO3)2 Ag+ NaNO3

Depth Ni profile analyses of XPS. The profile indicates that after Ni... | Download Scientific Diagram

Ethylene Dimerization and Oligomerization Using Bis(phosphino)boryl Supported Ni Complexes | Journal of the American Chemical Society

Overcoming Limitations in Decarboxylative Arylation via Ag–Ni Electrocatalysis | Journal of the American Chemical Society

SOLVED: 25.Which of the following would you expect to produce a reaction When mixed together? Ag(s) + Co(NOa)z(aq) Pb(s) Co(NOs)z(aq) Sn(s) + Ni(NOs)2(aq) Ni(s) + AgNOs(aq) Pb(NOs)zlaq) Co(NO3) (aq)

![The complex of [Co(NH3)5Br]Cl , the ionization isomer will give colour with AgNO3 . The complex of [Co(NH3)5Br]Cl , the ionization isomer will give colour with AgNO3 .](https://toppr-doubts-media.s3.amazonaws.com/images/7410843/66afa896-6332-4a2e-a0c9-acc6154c6ca9.jpg)