
Radical Mechanism of IrIII/NiII-Metallaphotoredox-Catalyzed C(sp3)–H Functionalization Triggered by Proton-Coupled Electron Transfer: Theoretical Insight | CCS Chem

Synthesis and catalytic activity of nickel(II) complexes containing NCN pincer ligands - ScienceDirect
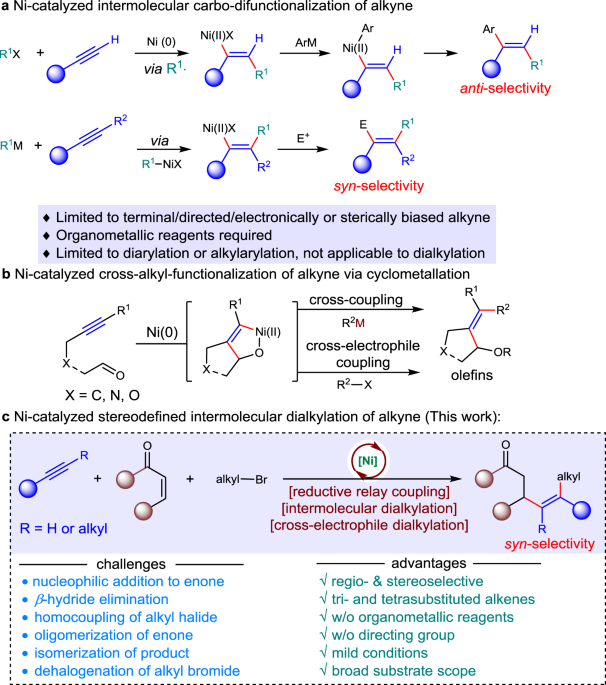
Ni-catalyzed regio- and stereo-defined intermolecular cross-electrophile dialkylation of alkynes without directing group | Nature Communications

Merging photoredox with nickel catalysis: Coupling of α-carboxyl sp3-carbons with aryl halides | Science
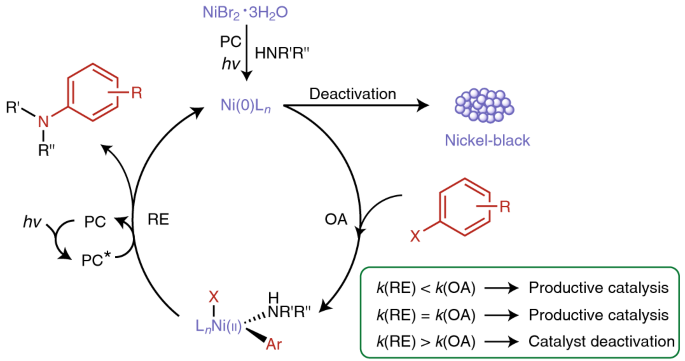
Overcoming limitations in dual photoredox/nickel-catalysed C–N cross-couplings due to catalyst deactivation | Nature Catalysis

Mechanisms of Nickel-Catalyzed Coupling Reactions and Applications in Alkene Functionalization | Accounts of Chemical Research
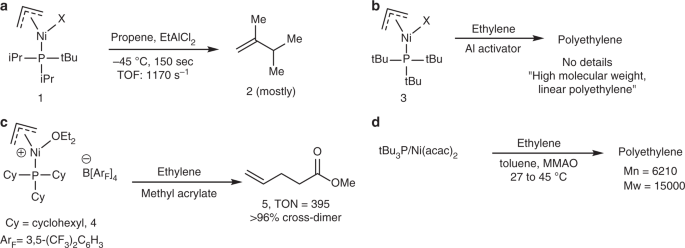
A highly active Ni(II)-triadamantylphosphine catalyst for ultrahigh-molecular-weight polyethylene synthesis | Nature Communications
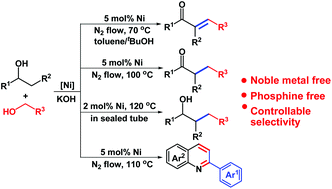
Reaction condition controlled nickel(ii)-catalyzed C–C cross-coupling of alcohols - Organic & Biomolecular Chemistry (RSC Publishing)

Ni(II)-Catalyzed Intramolecular C–H/C–H Oxidative Coupling: An Efficient Route to Functionalized Cycloindolones and Indenoindolones,ACS Catalysis - X-MOL

Ligand-Enabled Ni(II)-Catalyzed Hydroxylarylation of Unactivated Alkenes With Molecular Oxygen | Catalysis | ChemRxiv | Cambridge Open Engage
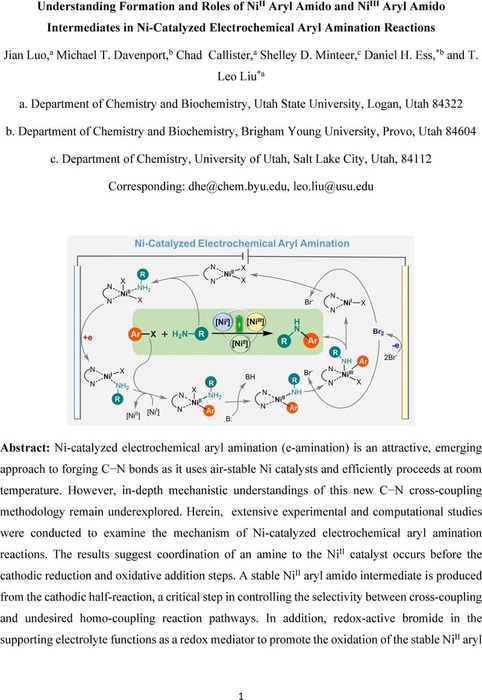
Understanding Formation and Roles of Ni(II) Aryl Amido and Ni(III) Aryl Amido Intermediates in Ni-Catalyzed Electrochemical Aryl Amination Reactions | Catalysis | ChemRxiv | Cambridge Open Engage
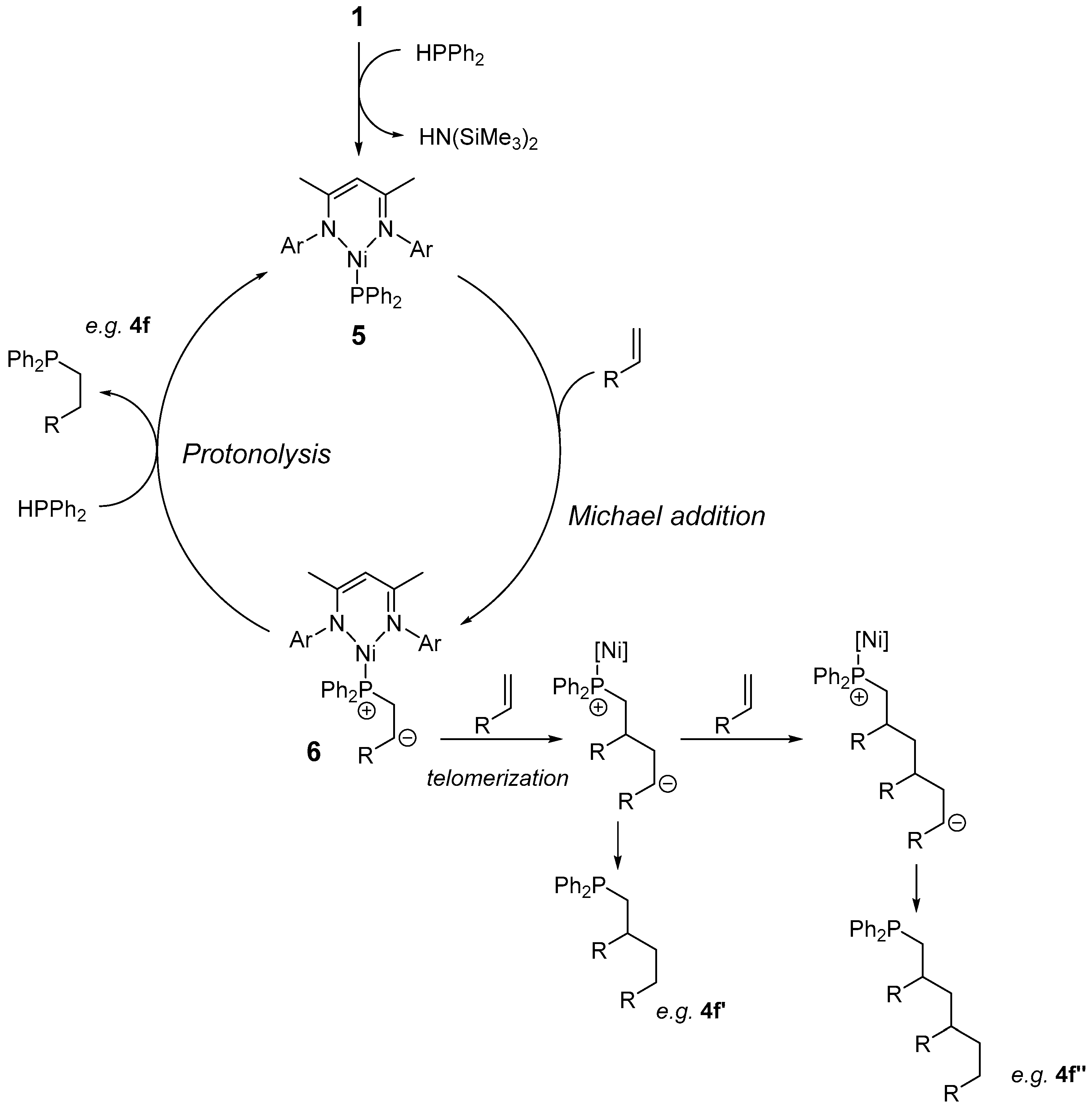
Inorganics | Free Full-Text | Room Temperature Ni(II) Catalyzed Hydrophosphination and Cyclotrimerization of Alkynes

Ni(II)-(σ-Aryl) Complex: A Facile, Efficient Catalyst for Nickel-Catalyzed Carbon−Nitrogen Coupling Reactions

A surprising mechanism lacking the Ni(0) state during the Ni(II)-catalyzed P–C cross-coupling reaction performed in the absence of a reducing agent – An experimental and a theoretical study

From Bis(silylene) and Bis(germylene) Pincer-Type Nickel(II) Complexes to Isolable Intermediates of the Nickel-Catalyzed Sonogashira Cross-Coupling Reaction | The Hartwig Group
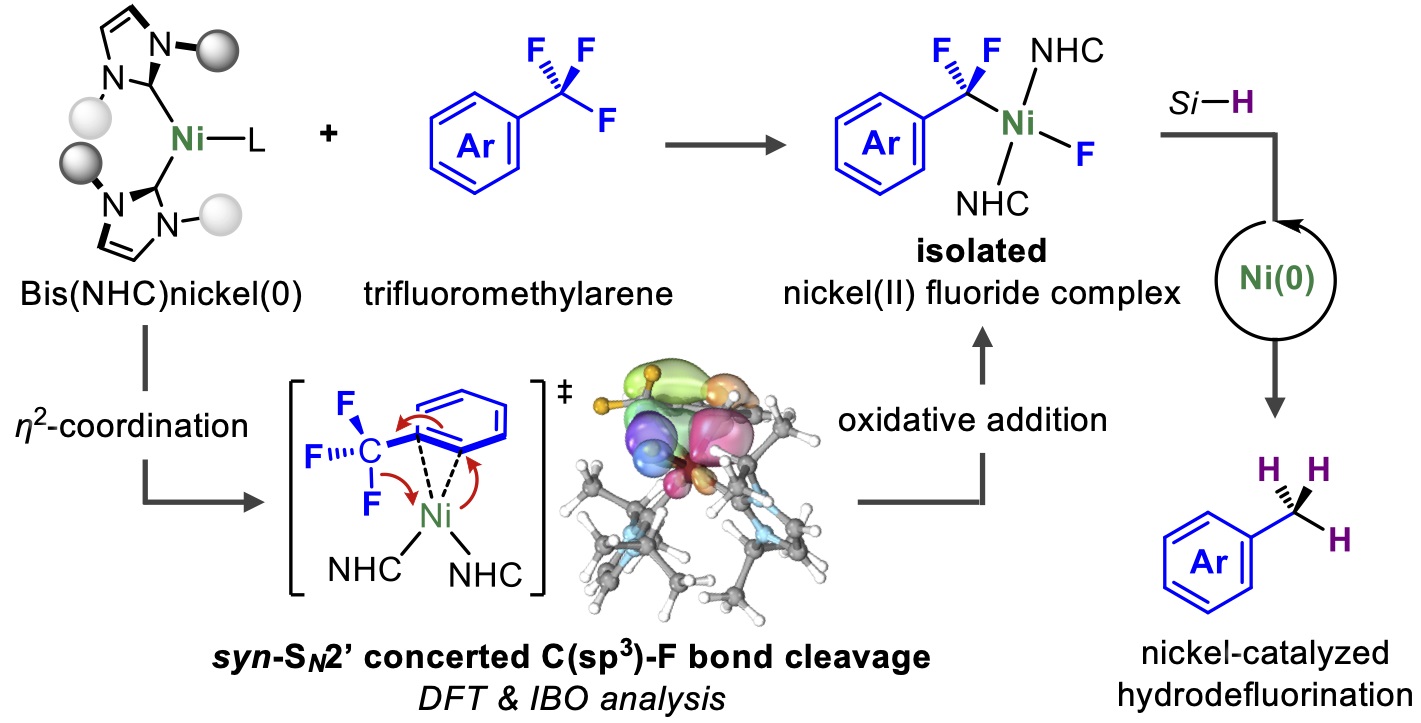




![PDF] Mechanisms of Nickel-Catalyzed Cross-Coupling Reactions | Semantic Scholar PDF] Mechanisms of Nickel-Catalyzed Cross-Coupling Reactions | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/3297cc29ee21e706a719bd5da81dca59c2ac6ab2/3-FigureI-1.png)
