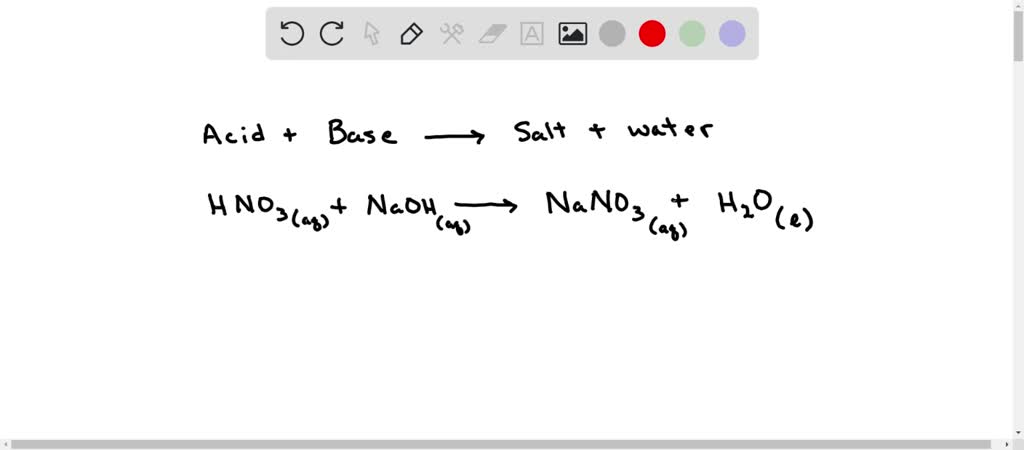
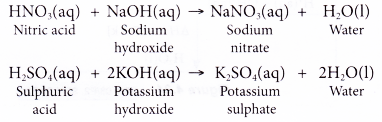Write fully balanced equation for the reaction of dilute nitric acid with the following chemical : Sodium hydroxide
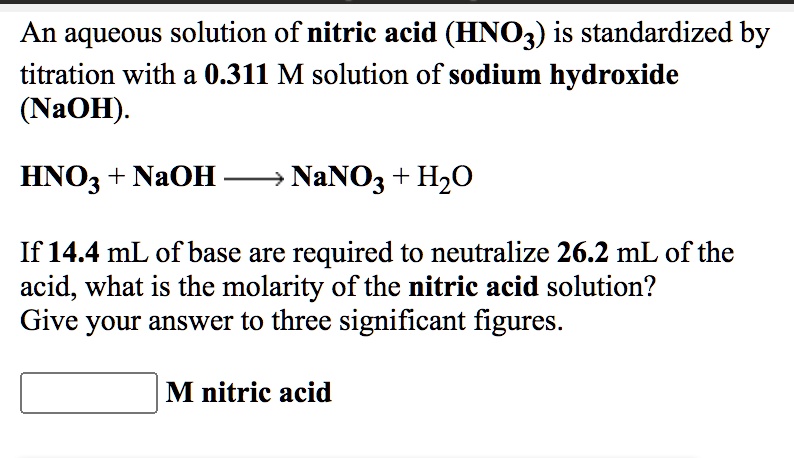
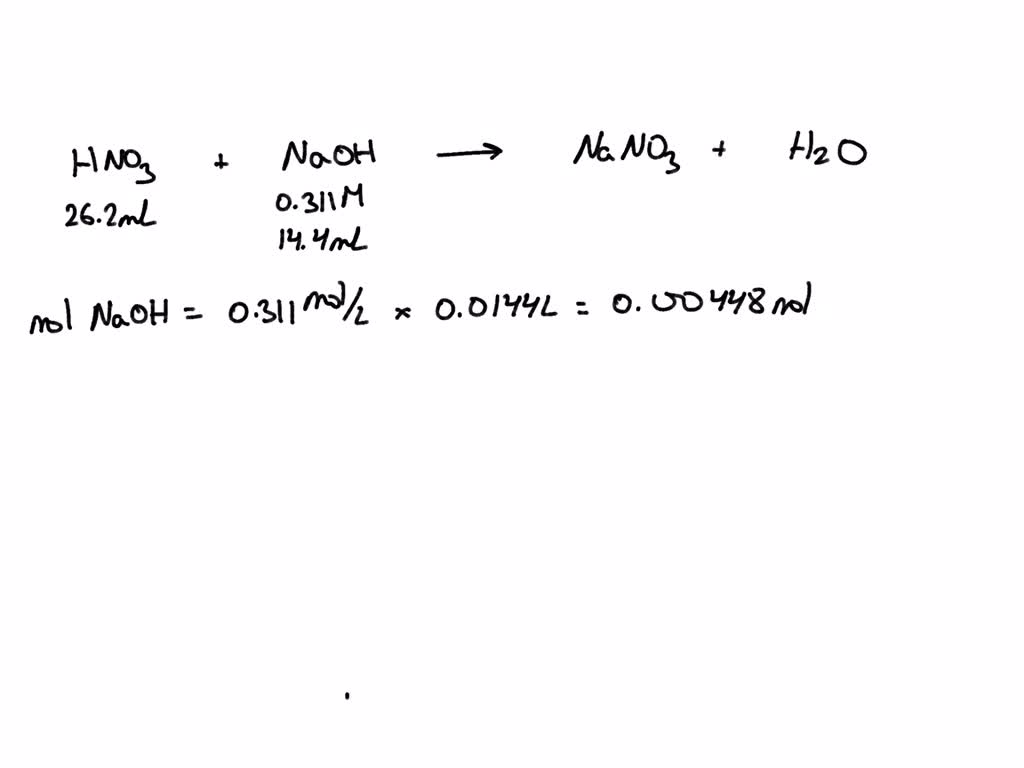
SOLVED: An aqueous solution of nitric acid (HNO3) is standardized by titration with a 0.311 M solution of sodium hydroxide (NaOH): HNOz + NaOH 77 NaNOz + H2O If14.4 mL of base
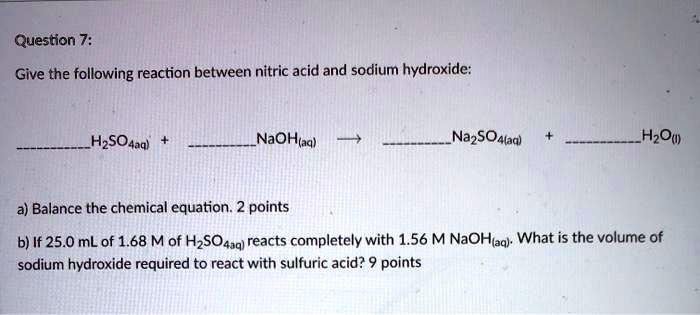
SOLVED: Question 7: Give the following reaction between nitric acid and sodium hydroxide: HzSOAaal NaOHtaq) NazSOAlaa) HzOu) a) Balance the chemical equation. 2 points b) If 25.0 mL of 1.68 Mof HzSO4uq)

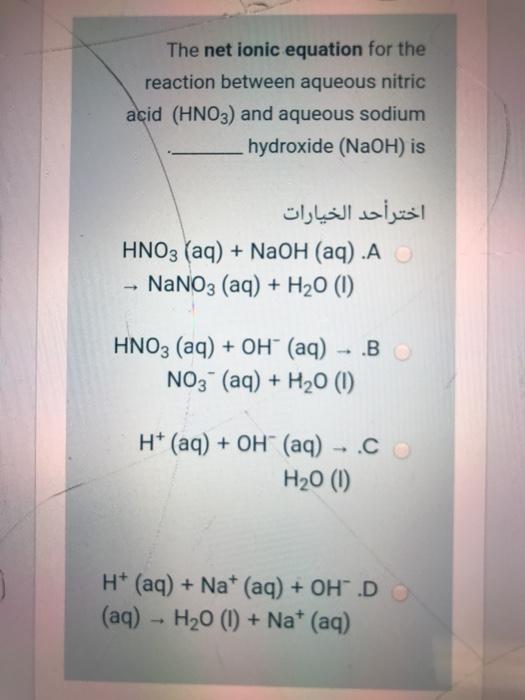
SOLVED: The net ionic equation for the reaction between aqueous nitric acid (HNO;) and aqueous sodium hydroxide (A) HNO; (aq) OH (aq) NO; (aq) HzO () (B) H* (aq) = OH- (aq) -
SOLVED: The reaction between nitric acid, HNO3(aq), and NaOH is shown below. HNO3(aq)(aq) + NaOH(aq) −→ NaNO3(aq) + H2O(l) Use the experimental data provided below and the molarity of the NaOH solution

HNO3 + NaOH = NaNO3 + H20 Balanced Equation||Nitric Acid + Sodium Hydroxide Balanced Equation - YouTube

The pH titration curves of nitric acid (C 0 a = 0.0002 mol l −1 ) being... | Download Scientific Diagram
Write balanced chemical equation for the following : 1. Reaction of nitric acid with sodium bicarbonate. - Sarthaks eConnect | Largest Online Education Community

Which of the following statements are correct ? (i) Boron reacts with concentrated HNO3 to form nitric oxide and boric acid (ii) Boron reacts with fused NaOH to form H2O2 and boric

H 2 SO 4 + Zn 1) Sulphuric acid + zinc 3) Nitric acid + sodium thiosulphate 2) Hydrochloric acid + magnesium 4) Hydrogen peroxide with catalyst Popular. - ppt download

Ionic equations A chemical equation shows the number of atoms and molecules of the reactants and products. Also shows physical state of reactants and products. - ppt download