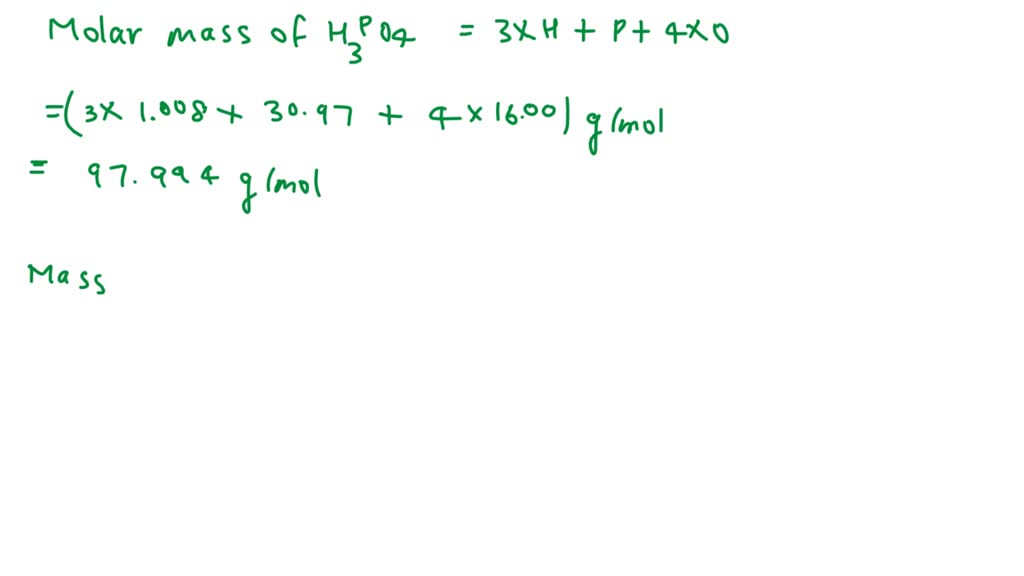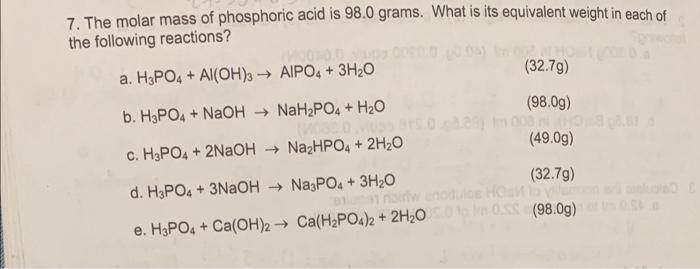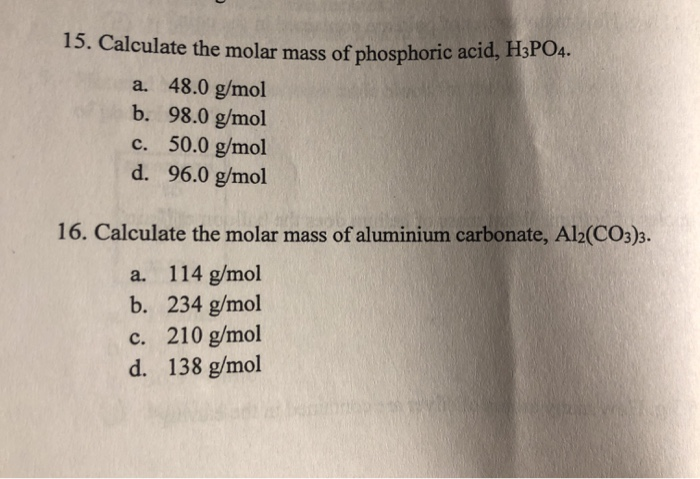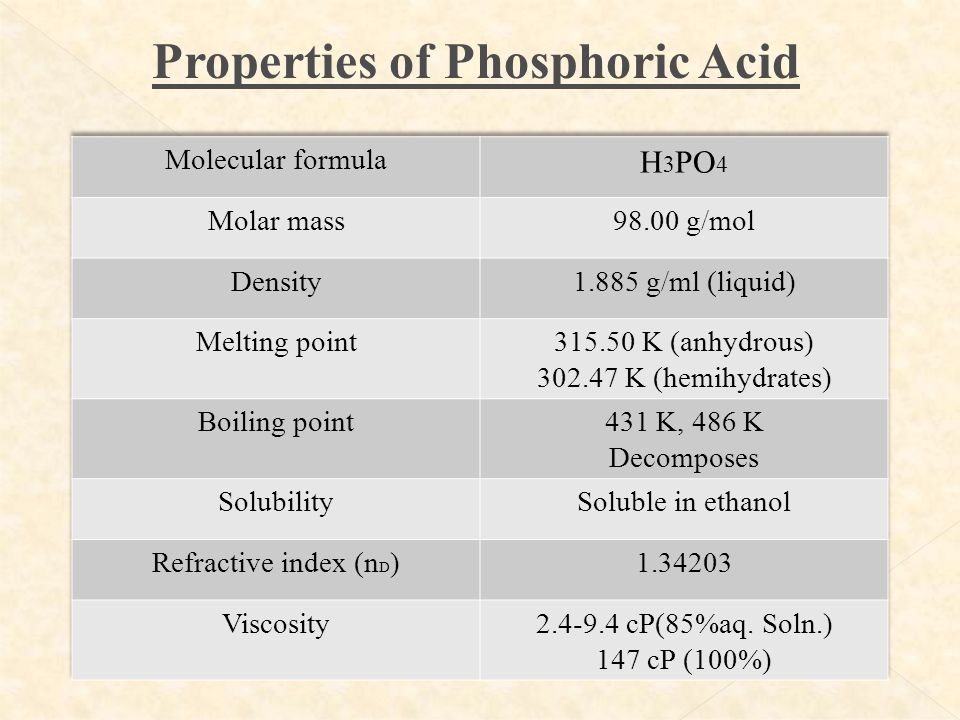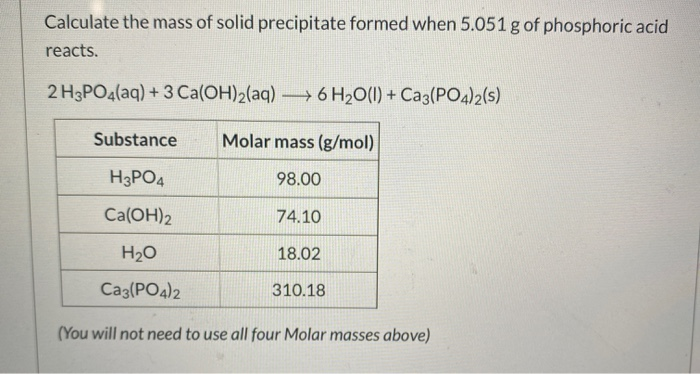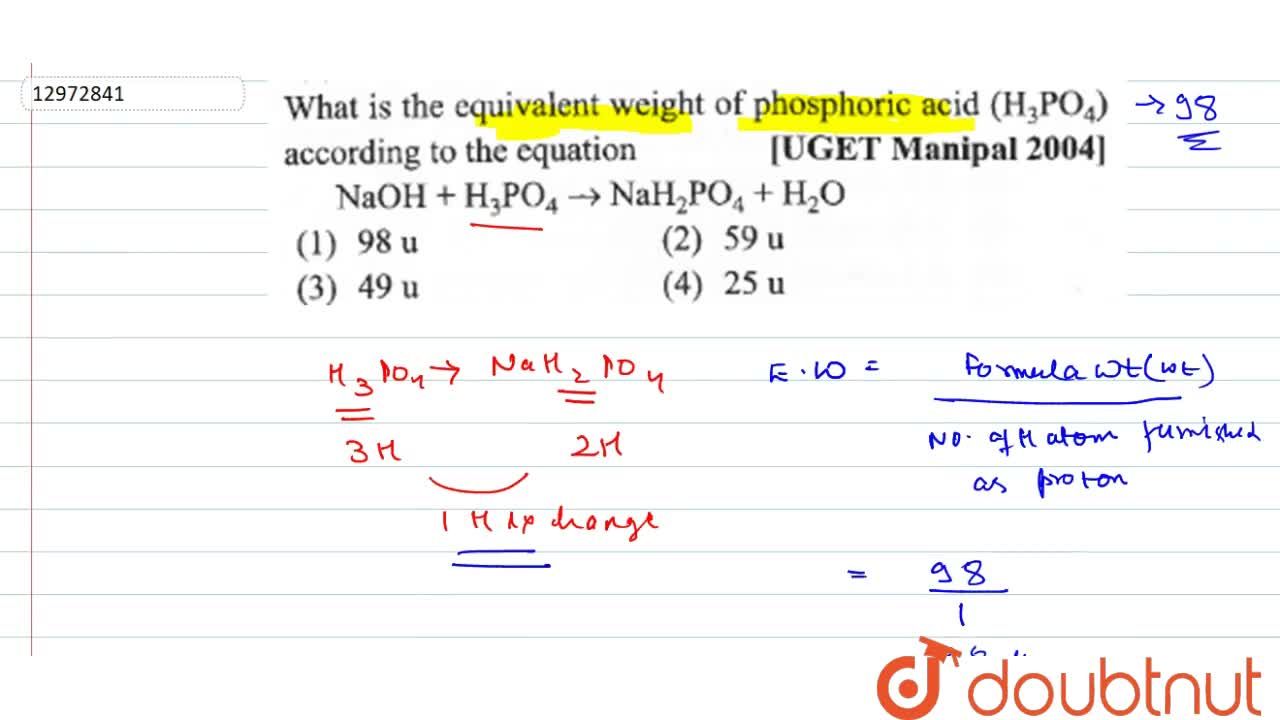
What is the equivalent weight of phosphoric acid (H(3) PO(4)) according to the equation NaOH + H(3) PO(4) rarr NaH(2) PO(4) + H(2)O

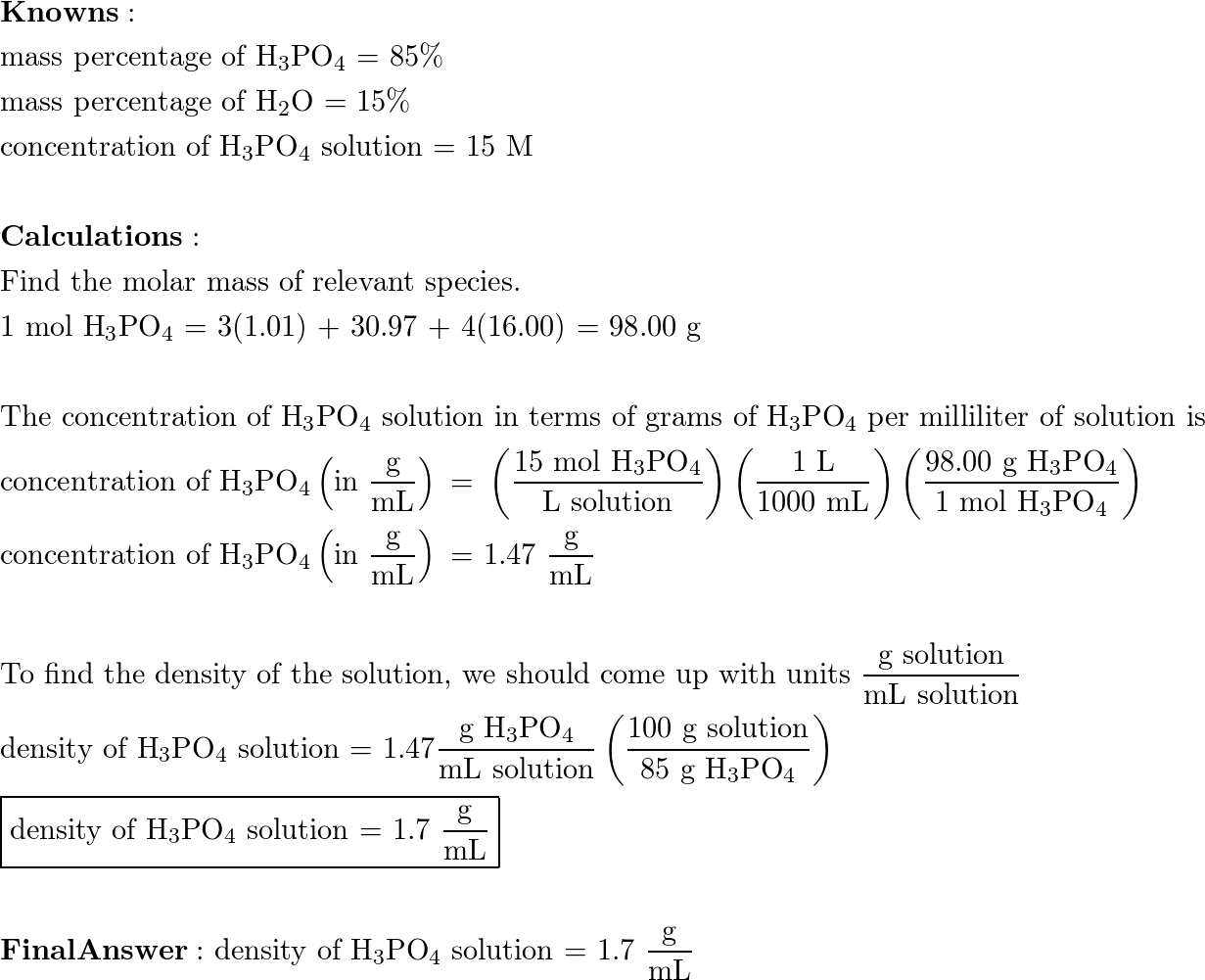
OneClass: Calculate molality of a 35.4% (by mass) aqueous solution of phosphoric acid (H3PO4). The mo...

The molecular weight of phosphoric acid, H 3 PO 4 , is 97.99 * g/(mol) What is the mass, in grams, of - Brainly.com
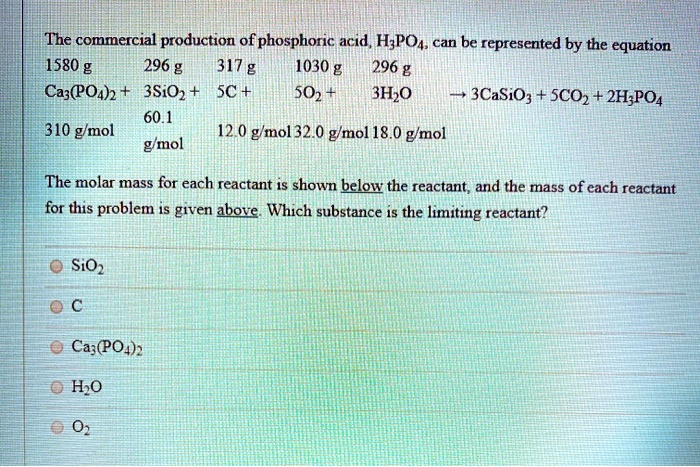
SOLVED: The commercial production of phosphoric acid, HzPOA can be represented by the equation 1580 g 296 g 317 g 1030 g 296 g Caz(PO4)2 3Si02 SC + 502 3HzO 3CaSiOg SCO2 +

SOLVED: The morality of a 30.5% by mass aqueous solution of phosphoric acid H3PO4 is. molar mass of H3PO4 = 97.99g/mol

What is the equivalent weight of phosphoric acid `(H_(3) PO_(4))` according to the equation - YouTube