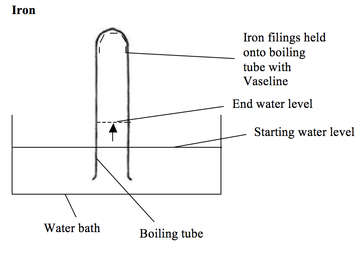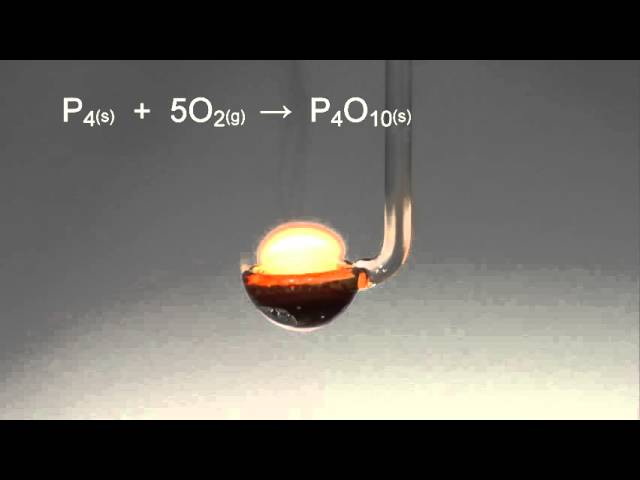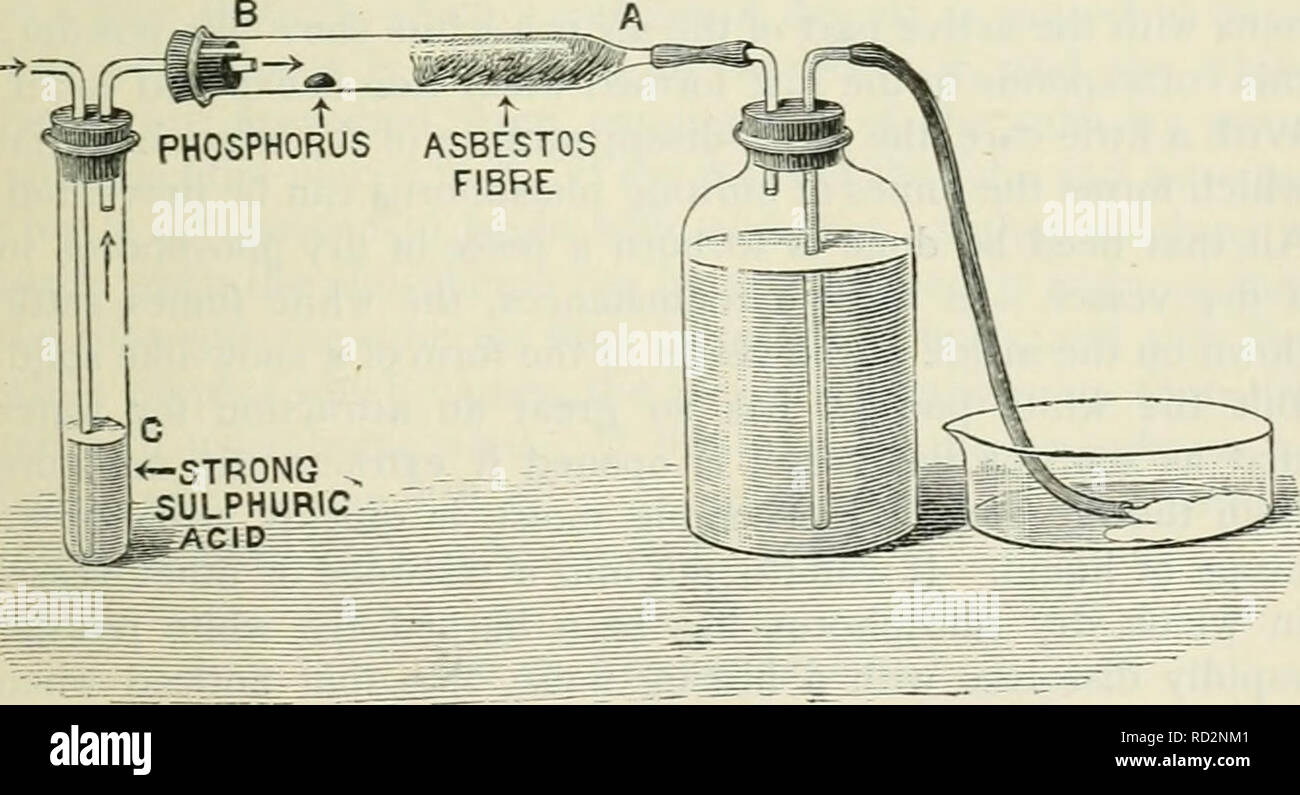
Elementary physics and chemistry: second stage. Science. BURNING PHOSPHORUS IN AIR. 109 powder is deposited upon the sides of the cylinder. When the phosphorus has ceased to burn, hft up the
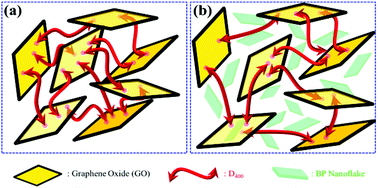
Graphene oxide/black phosphorus nanoflake aerogels with robust thermo-stability and significantly enhanced photothermal properties in air - Nanoscale (RSC Publishing)
Animal activities; a first book in zoo?logy. Zoology; Animal behavior. 28 ANIMAL ACTiyiTIES. piece of phosphorus. Float the cork on water in a soup-plate and light the phosphorus, at the same

Enhancing thermal oxidation and fire resistance of reduced graphene oxide by phosphorus and nitrogen co-doping: Mechanism and kinetic analysis - ScienceDirect
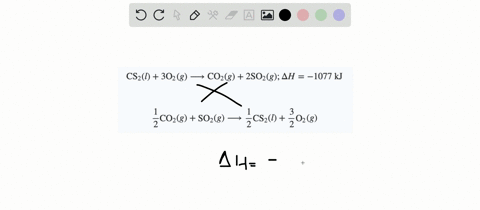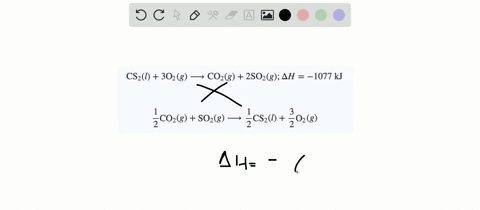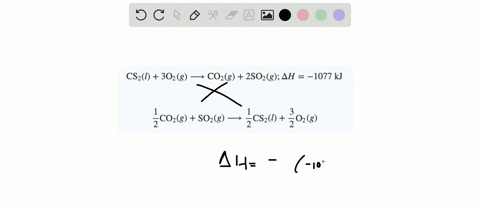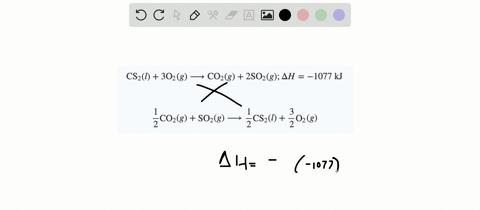
SOLVED:When white phosphorus burns in air, it produces phosphorus(V) oxide. P4(s)+5 O2(g) ⟶P4 O10(s) ; ΔH=-3010 kJ What is ΔH for the following equation? P4 O10(s) ⟶P4(s)+5 O2(g)
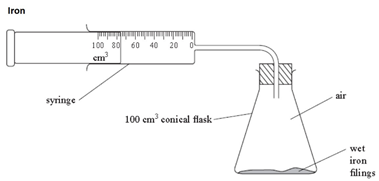
2:10 understand how to determine the percentage by volume of oxygen in air using experiments involving the reactions of metals (e.g. iron) and non-metals (e.g. phosphorus) with air - TutorMyself Chemistry

Translate the following statement into chemical equations and then balance the equations: (i) Phosphorus burns in oxygen to give phosphorus pentoxide. (ii) Aluminium metal replaces iron from ferric oxide, Fe(2)O(3), giving aluminium
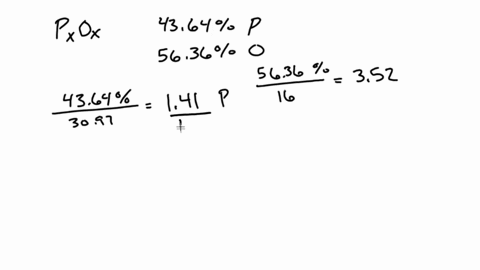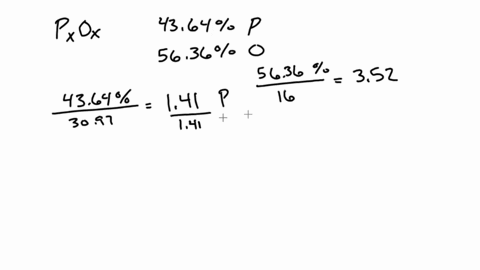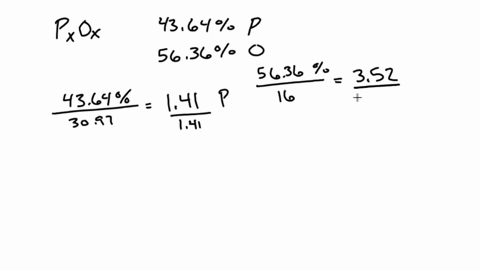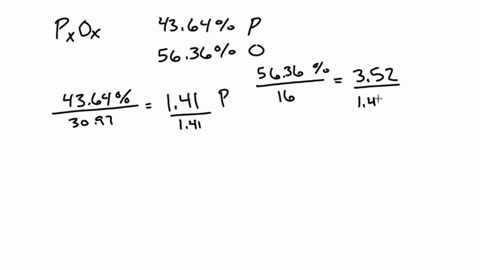
SOLVED:A 1.45-g sample of phosphorus burns in air and forms 2.57 g of a phosphorus oxide. Calculate the empirical formula of the oxide.