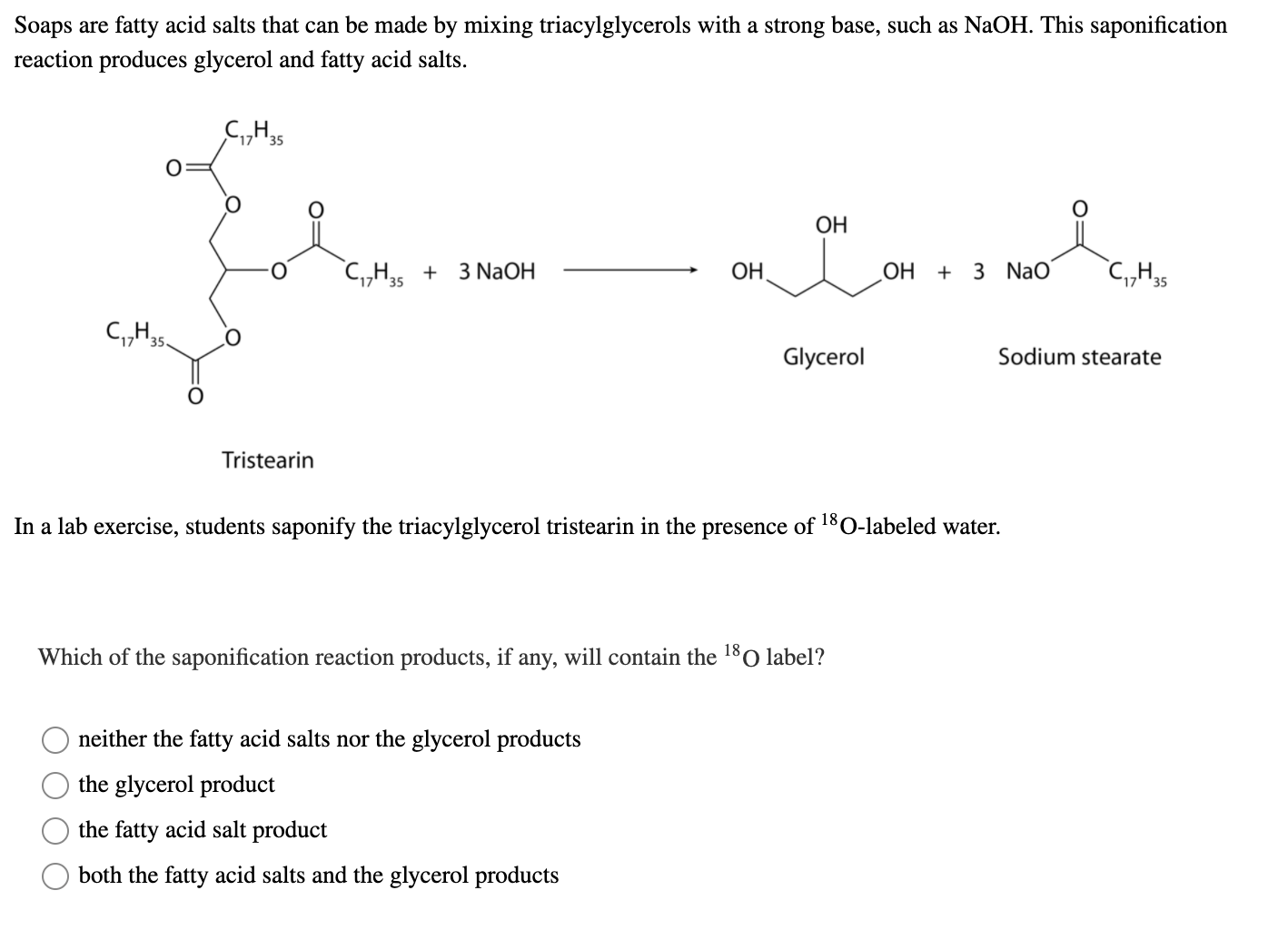
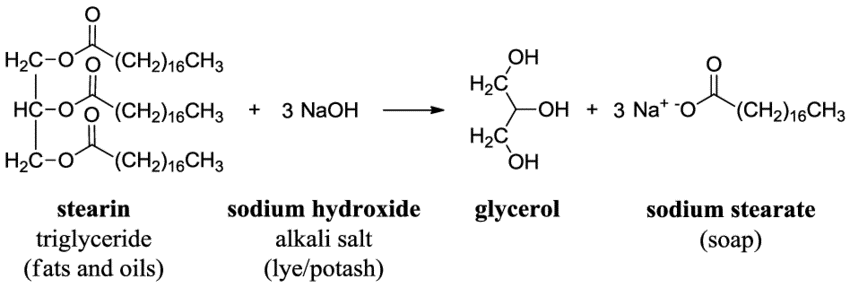
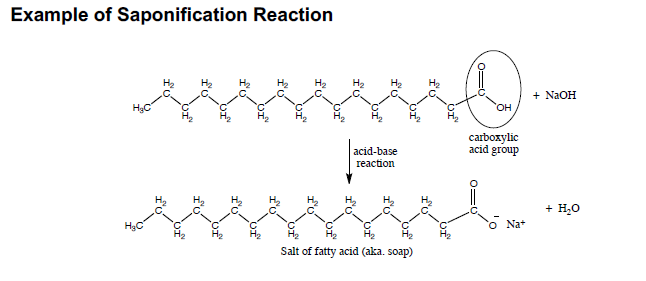
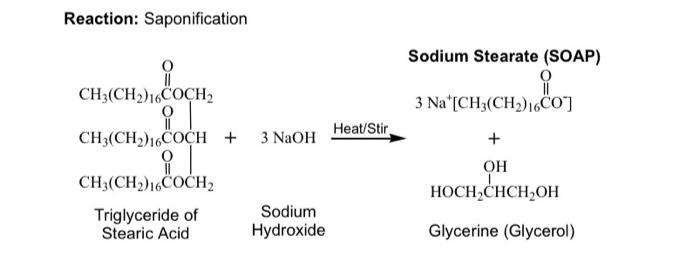
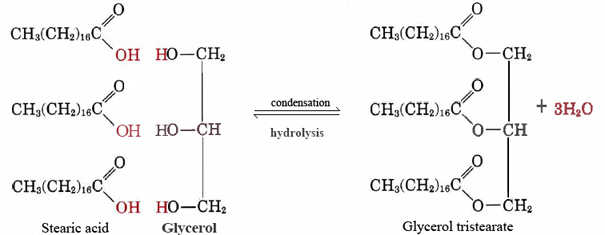
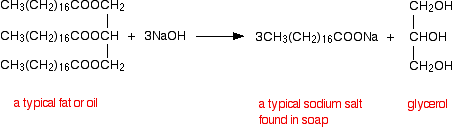
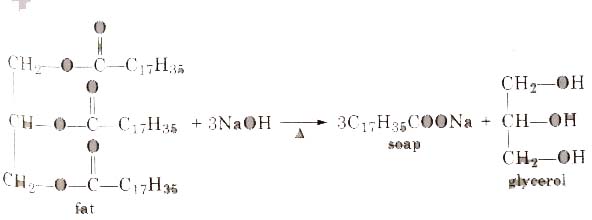Statement I: A triester of glycerol with stearic acid on boiling with `Aq`. `NaOH` gives solid cake - YouTube

SOLVED: Calculate the theoretical yield of sodium stearate (soap). 500 g triglyceride of stearic acid. We have an atom economy of 90.9%. The process is triglyceride of stearic acid + 3 Sodium
Stearic acid sodium salt, CAS No. 822-16-2 | Fatty acids and derivatives | Oils and Greases | Natural & Reference Materials | Chemicals | Carl Roth - International

EP0294010A1 - Process and apparatus for the continuous production of transparent soap - Google Patents

Write the equation desribing the formation of an ester from stearic acid and ethanol? | Homework.Study.com

EP0294010A1 - Process and apparatus for the continuous production of transparent soap - Google Patents

Statement I: A triester of glycerol with stearic acid on boiling with `Aq`. `NaOH` gives solid cake - YouTube

Mechanism of surface modification of jute fibers (JFs) by different... | Download Scientific Diagram