Dear Dr. Woodcock, I am writing to bring to your attention a Warning Letter that Vanda received from the FDA on October 22, whic

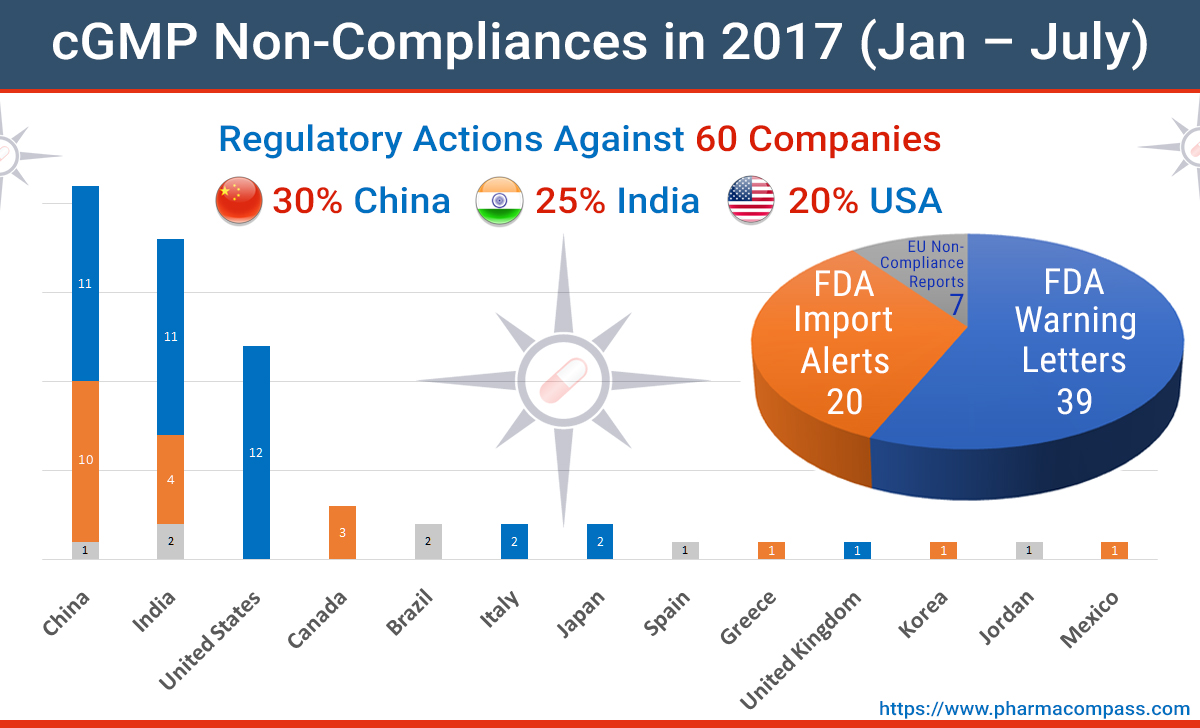
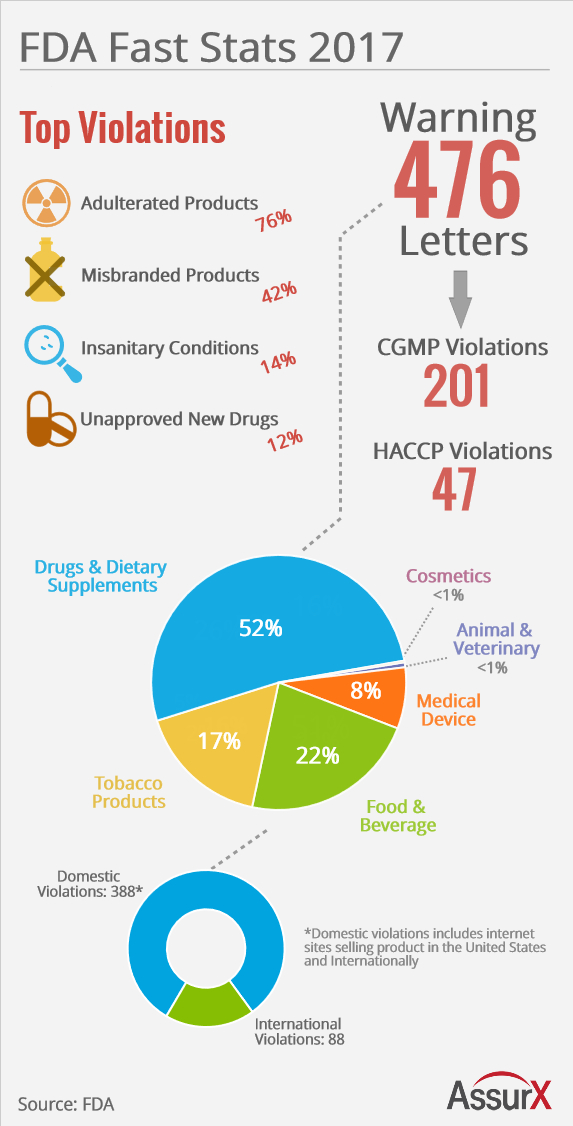
2016 — A year of data integrity issues and pharma non-compliances - Victimes d'Effets indésirables Graves ENDOXAN Cyclophosphamide Fatal adverse effects