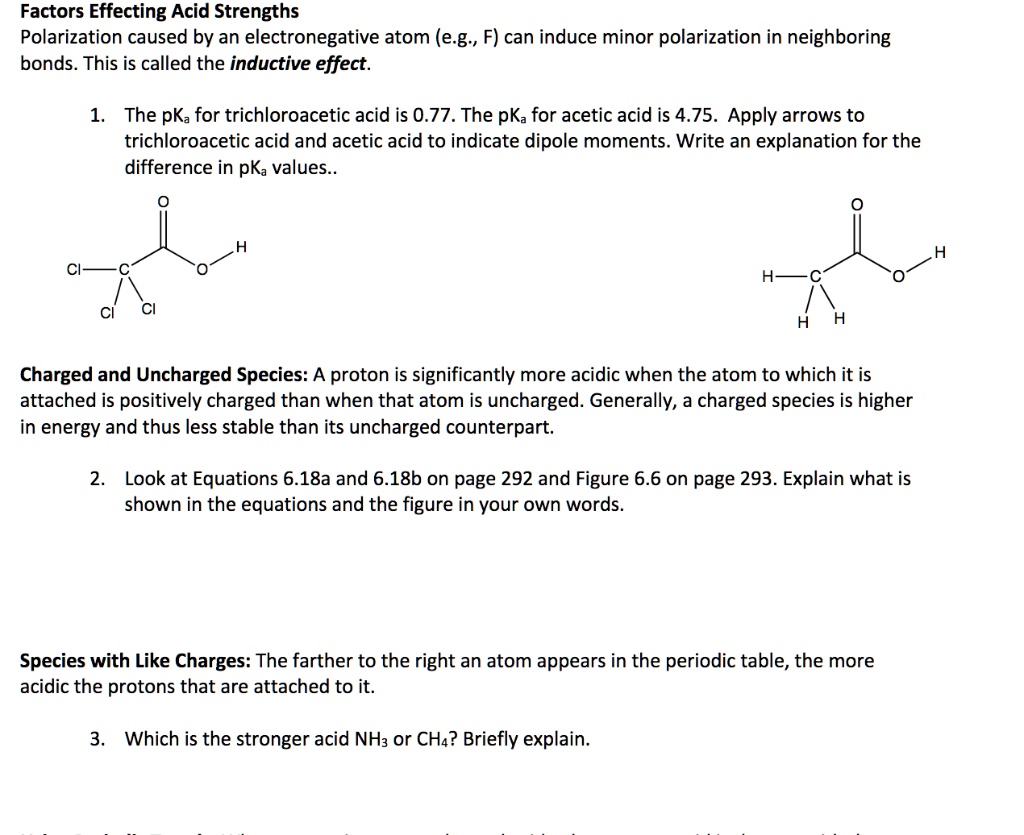
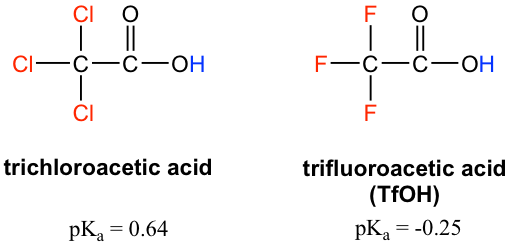
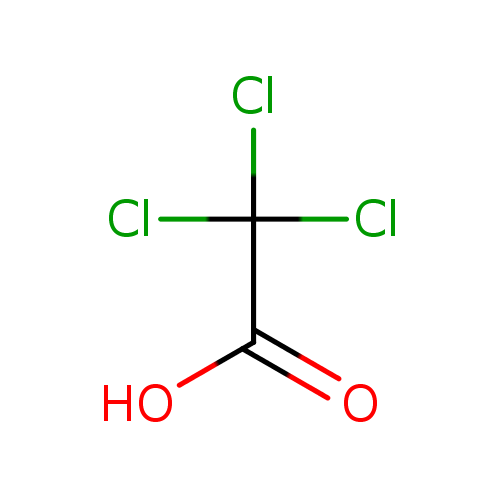
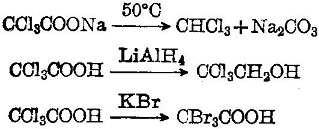
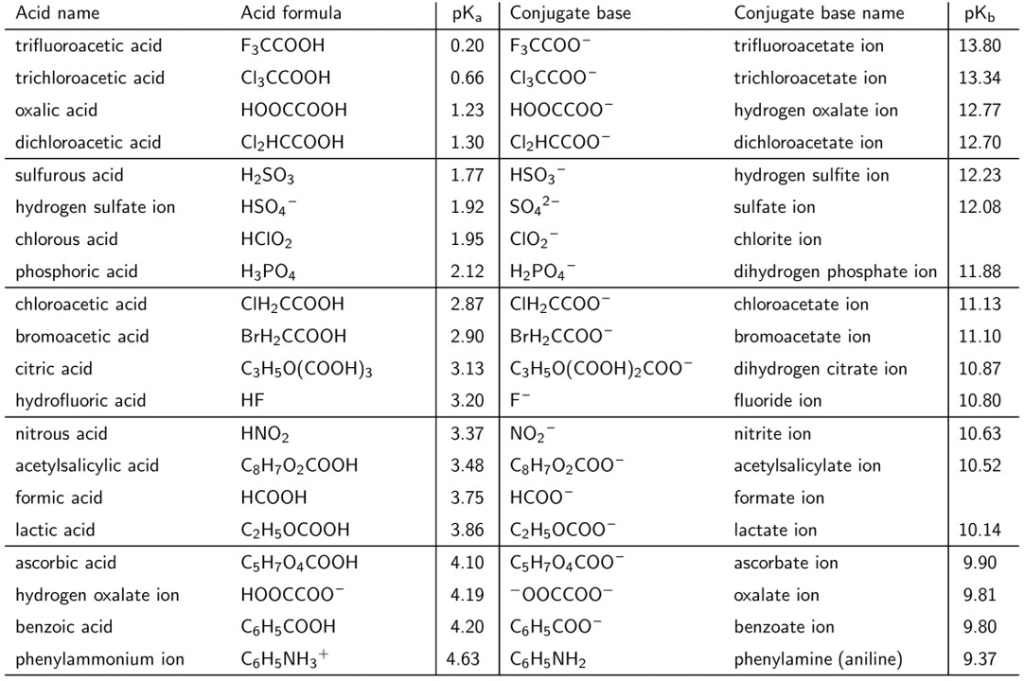
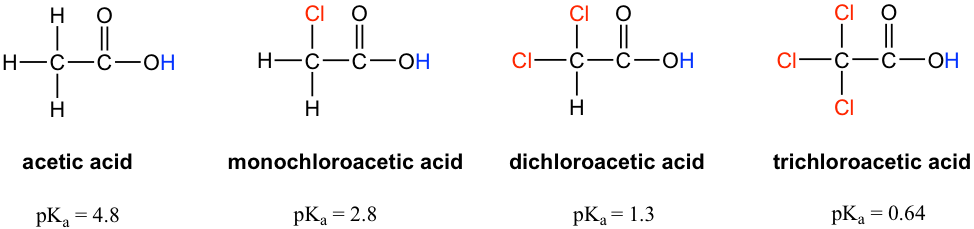
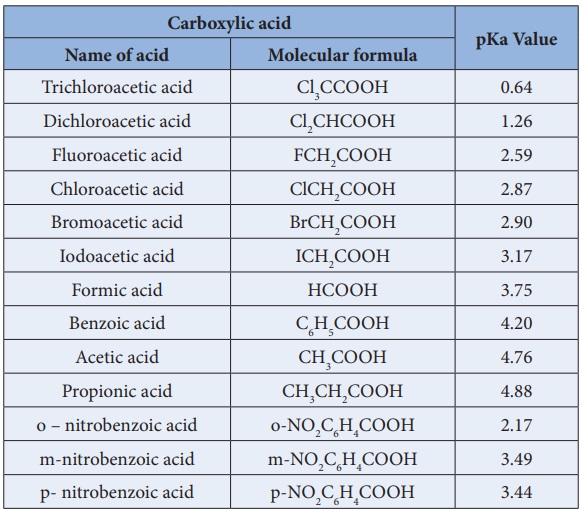
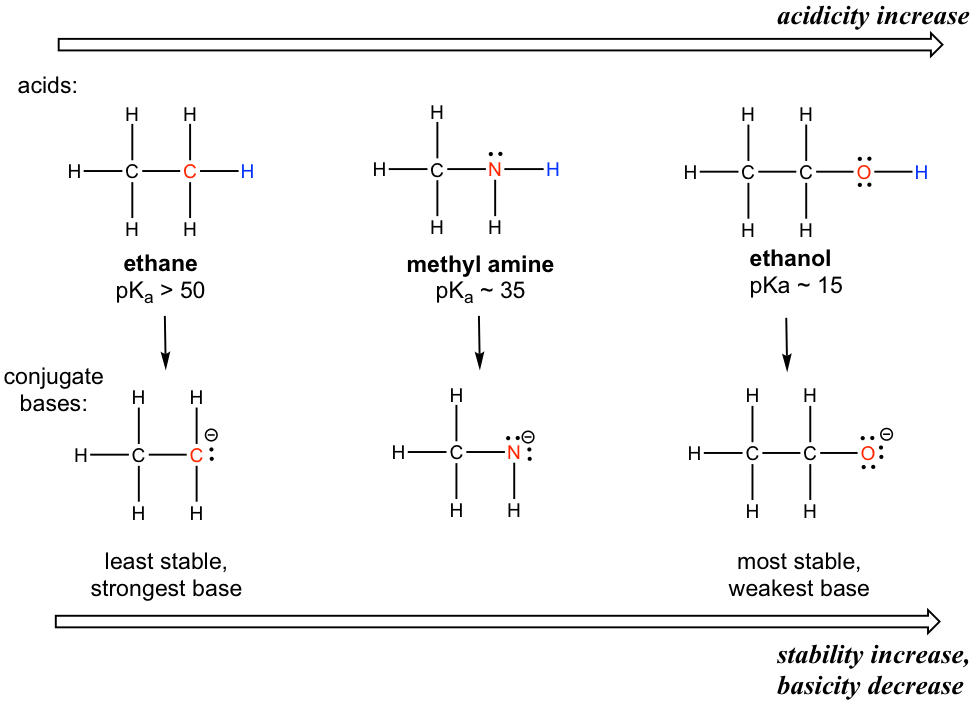
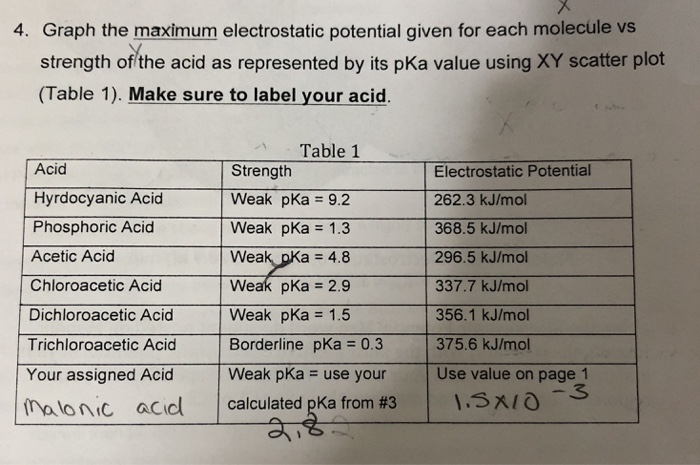
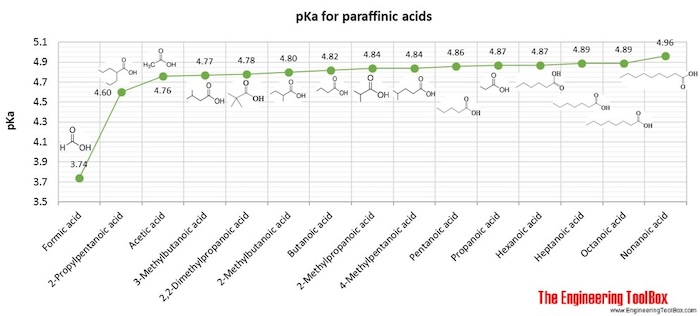
The pKa values of acetic acid and trichloroacetic acid are 4.7 and 0.7, respectively. Which is the stronger acid and what are the corresponding dissociation constants? - ECHEMI

SOLVED: Warts beware. The pKa of acetic acid is 4.76 and the pKa of trichloroacetic acid, which is used to remove warts, is 0.7. Calculate the dissociation constant of each acid. Which
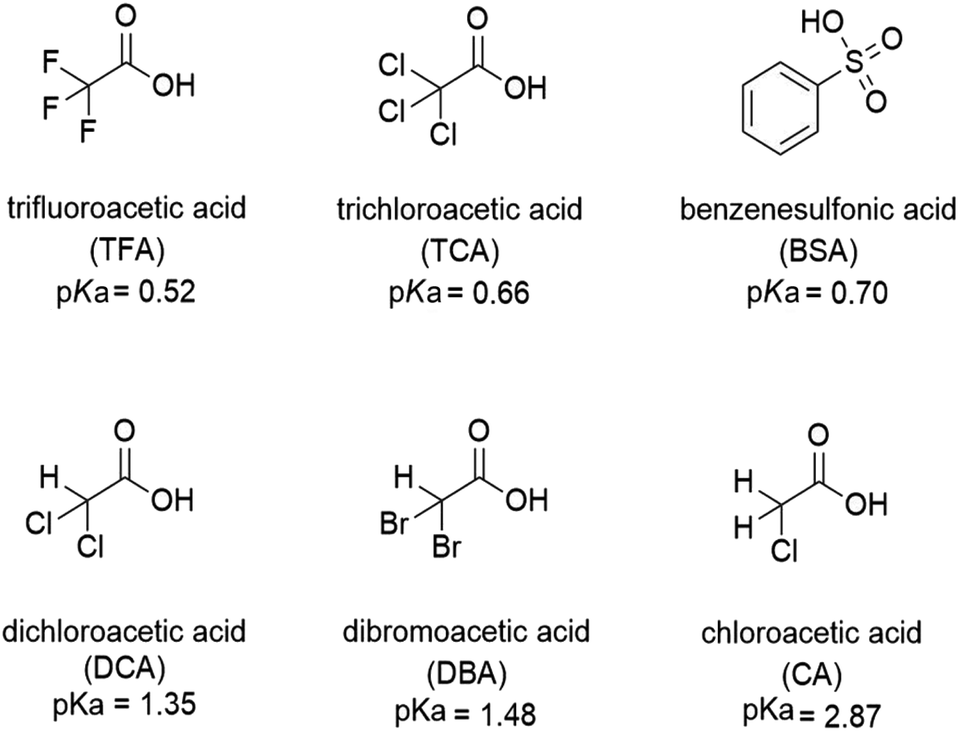
Fluorescence enhancement of quinolines by protonation - RSC Advances (RSC Publishing) DOI:10.1039/D0RA04691D

How to determine the strength of facial peels: debunking the myths and to determine how strong different peelings really are - nunii