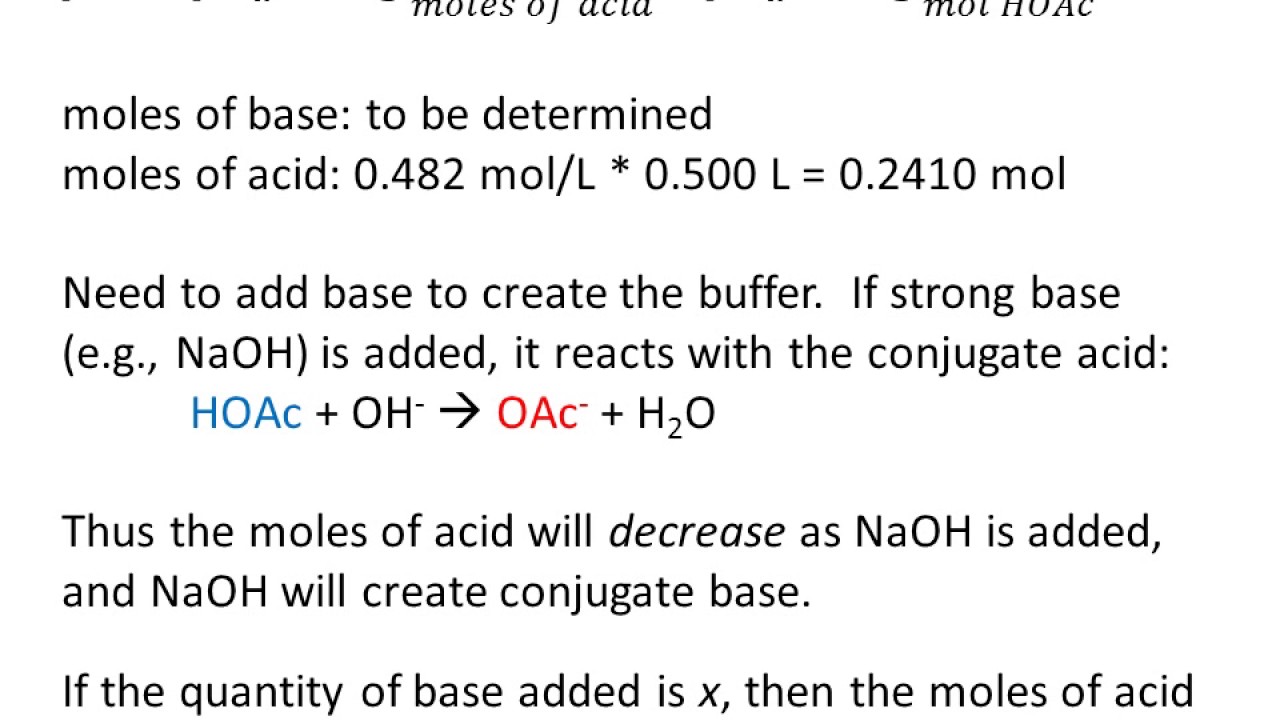
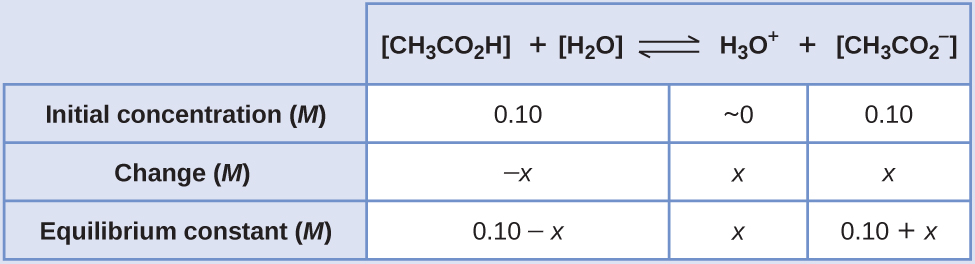
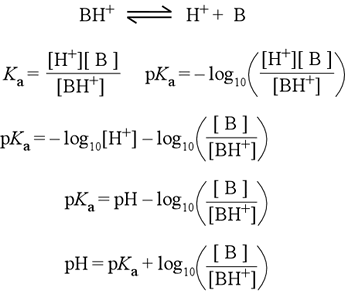If a buffer solution is 0.200 M in a weak acid (Ka = 1.6 x 10^-5) and 0.480 M in its conjugate base, what should the pH be? | Homework.Study.com

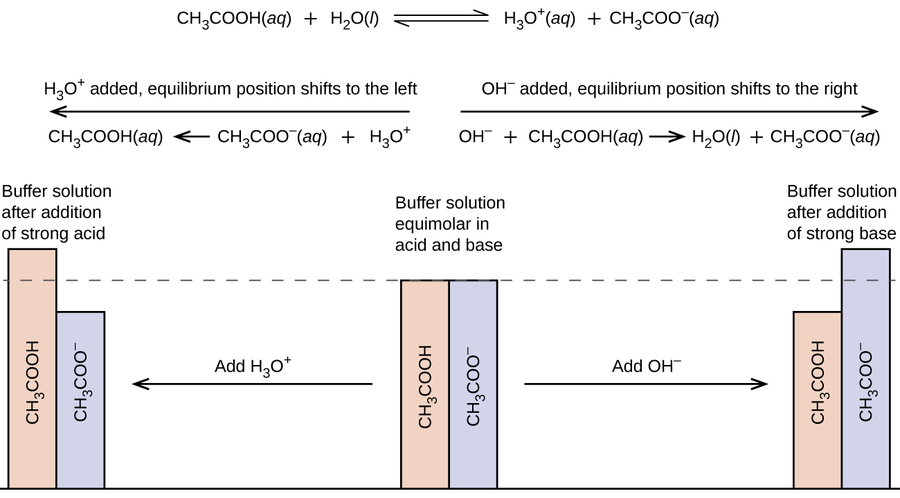
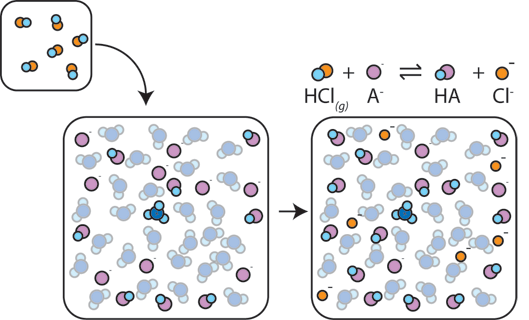
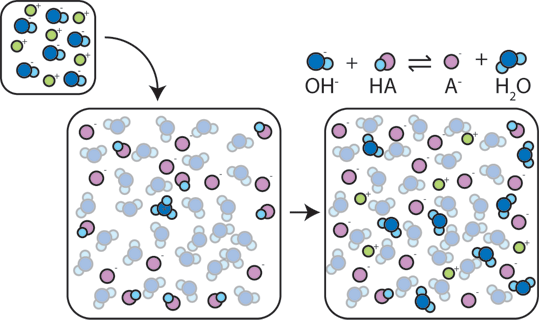
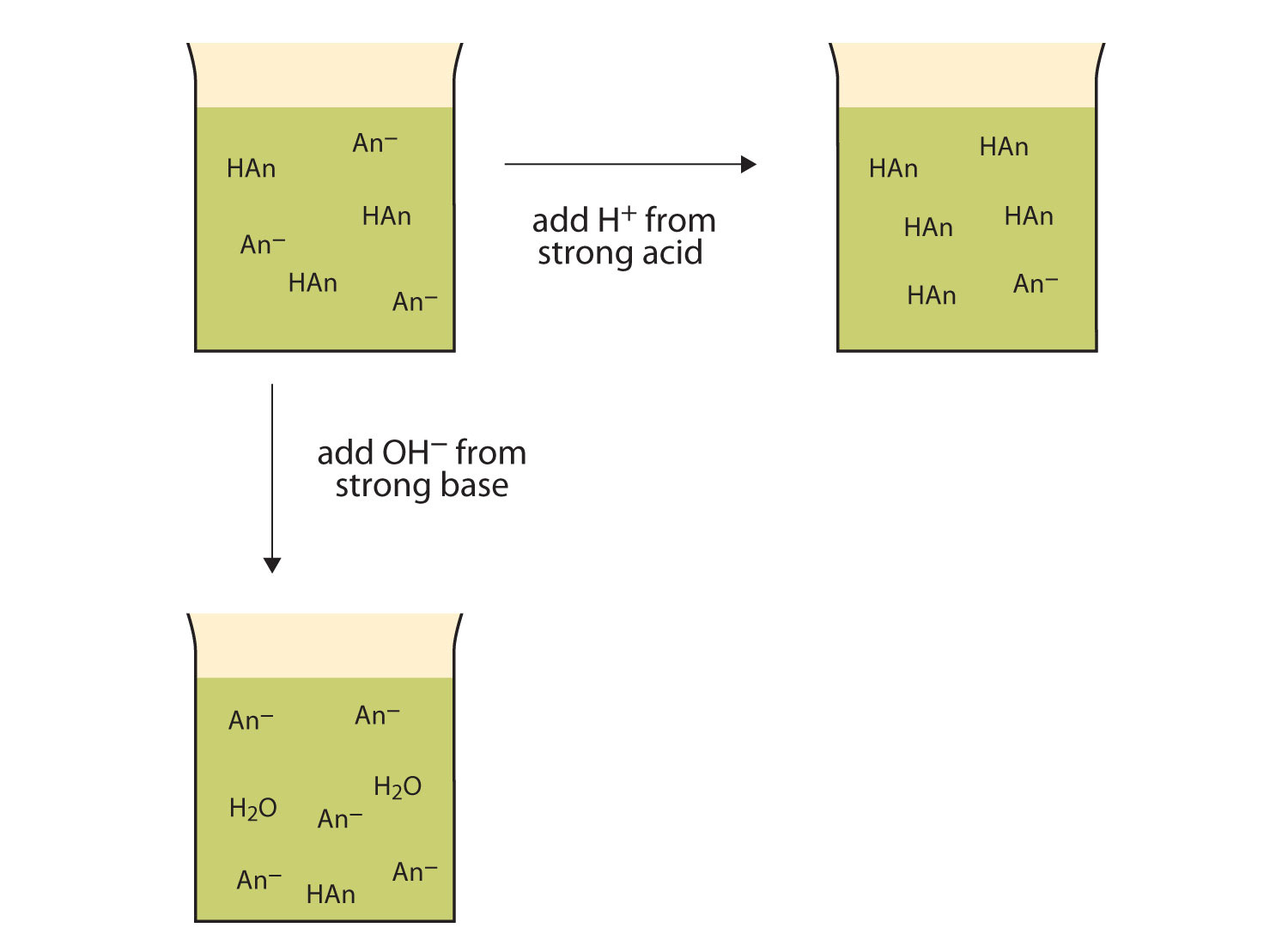
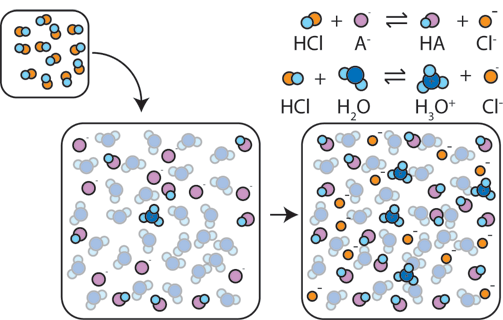
Yexel Exams - Buffer solutions are used to reduce pH fluctuations associated with introduction of small amounts of strong acids or bases. Typical buffer solutions are composed of a weak acid or
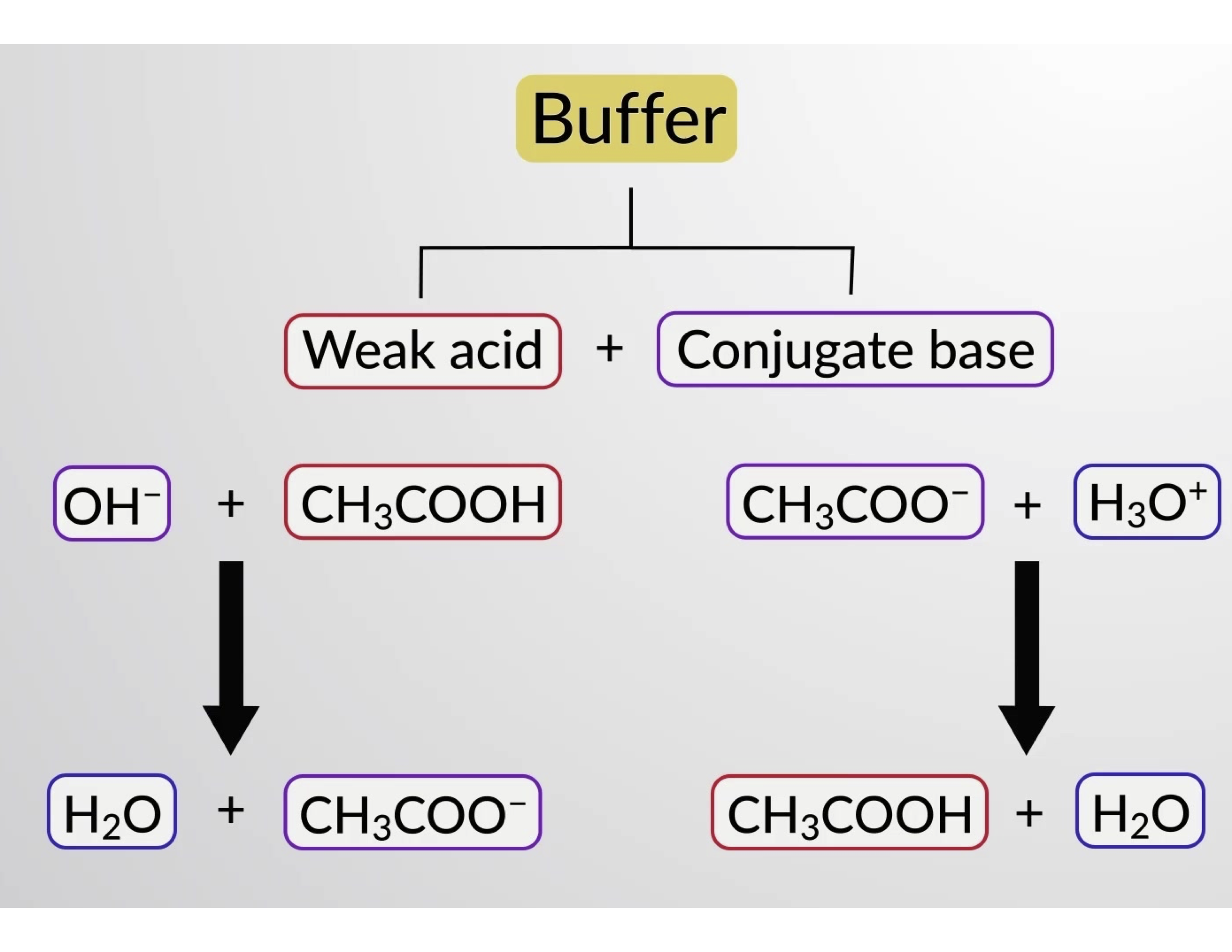
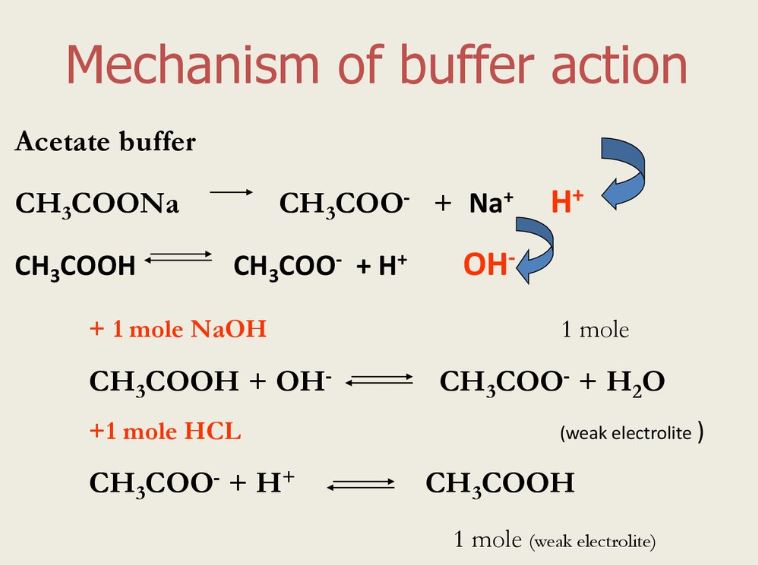
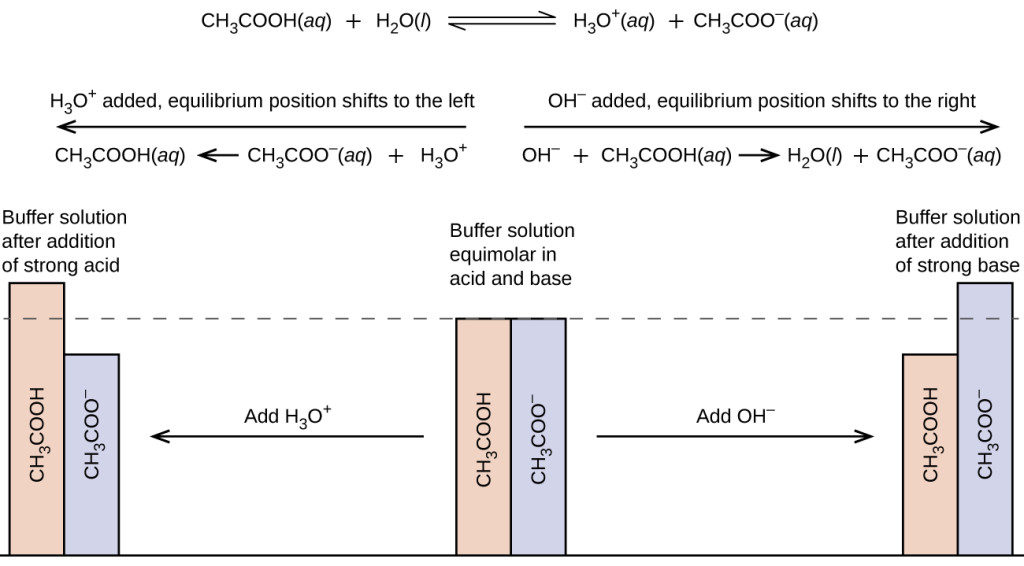
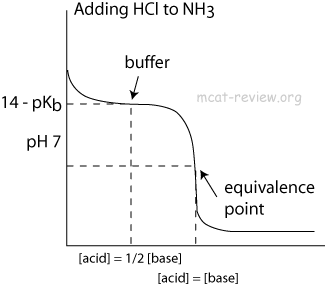
Why is an acidic buffer solution most effective when the concentrations of acid and base are the same? - Quora

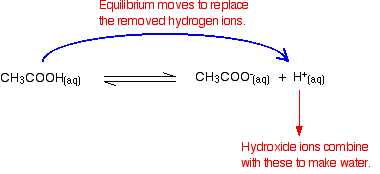
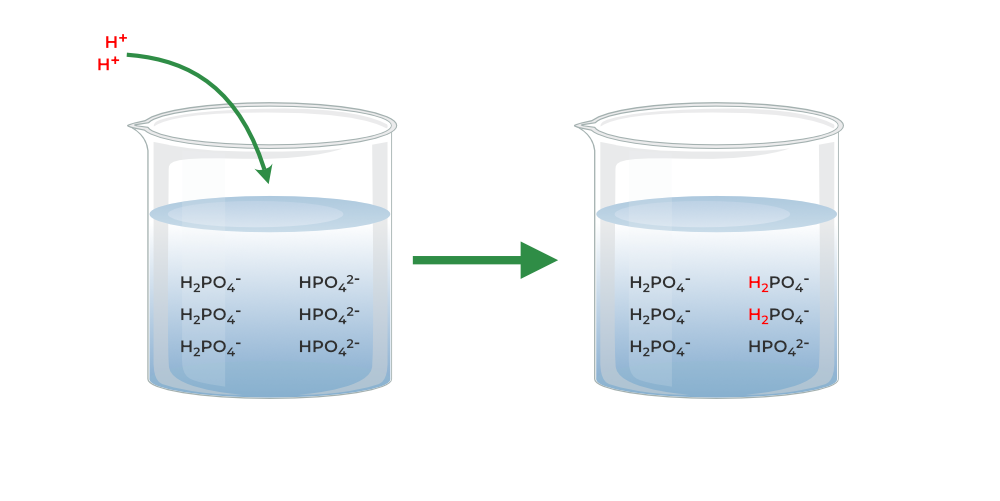
Buffer Region - What is a Buffer Region, Relationship between Titration and Buffer Region and How do Buffers Work along with FAQs