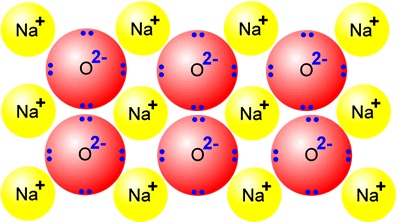
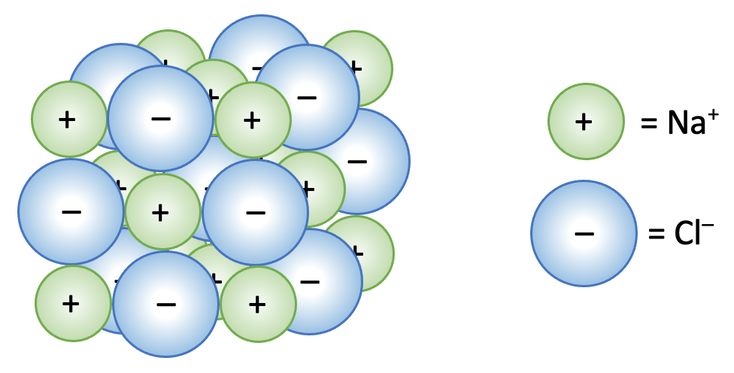
Starter- Copy the paragraph (Correct the mistakes) Ionic substances have low melting points and high boiling points. This is because they are held together. - ppt download

Encapsulation of Phase-Changing Eutectic Salts in Magnesium Oxide Fibers for High-Temperature Carbon Dioxide Capture: Beyond the Capacity–Stability Tradeoff | ACS Applied Materials & Interfaces
Which would you expect to have a higher melging point, magnesium oxide or magnesium fluoride ? Explanin your reasoning.

Question Video: Explaining Why the Melting Point of the Period Three Metals Increases from Group 1 to Group 13 | Nagwa
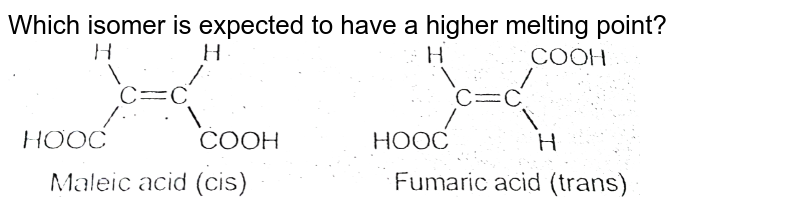
Which would you expect to have a higher melting point, magnesium oxide or magnesium fluoride? Explain your reasoning.

Survey of Period 3 element trends bonding, structure, oxidation states, formulae of oxides, chlorides, hydrides, reactions with oxygen, chlorine, water, acids, alkalis, isoelectronic series of ions atoms revision notes

Why magnesium oxide has higher melting point tan magnesium fluoride - Chemistry - The s-Block Elements - 13064067 | Meritnation.com

inorganic chemistry - Why is the melting point of magnesium oxide higher than aluminium oxide? - Chemistry Stack Exchange
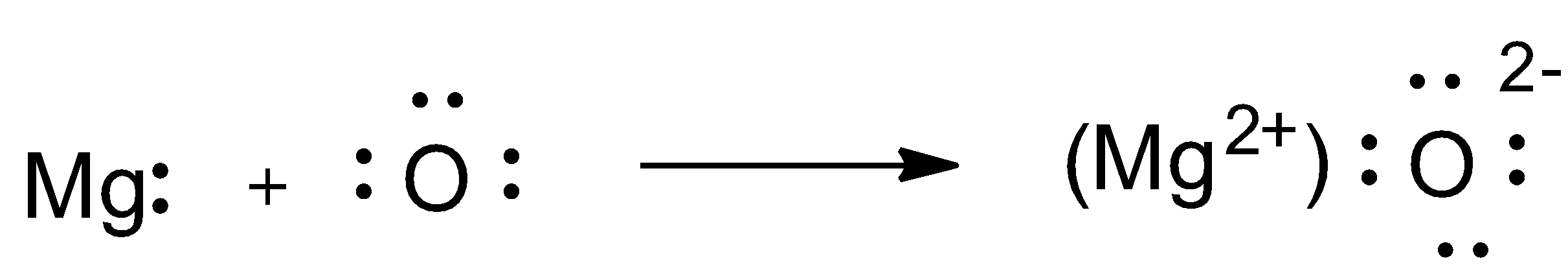
A) What are ionic compounds? Show the formation of magnesium oxide.(B) Among Covalent and ionic compounds which will have higher melting and boiling point and why?

Phase Transformations and Metallization of Magnesium Oxide at High Pressure and Temperature | Science