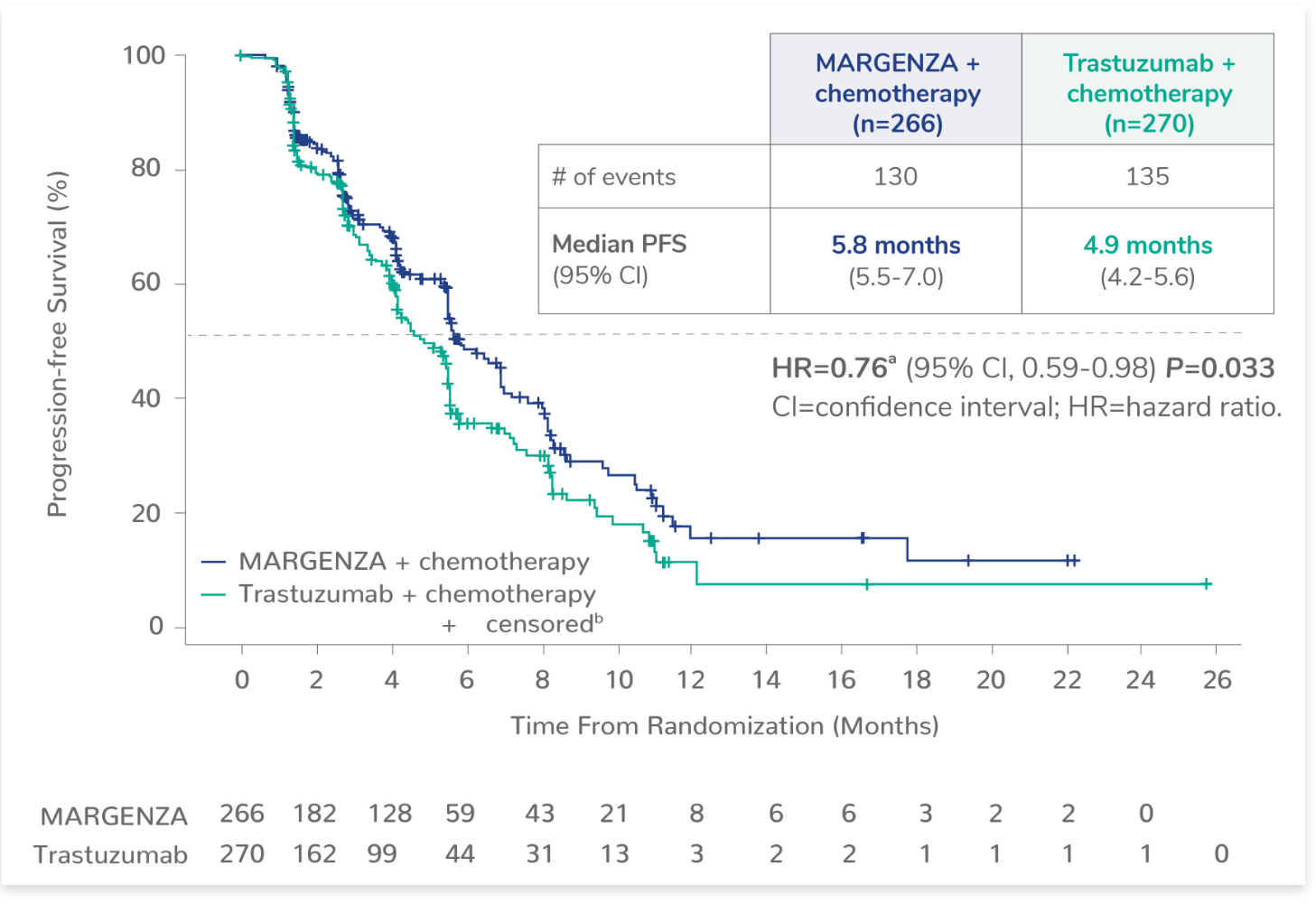
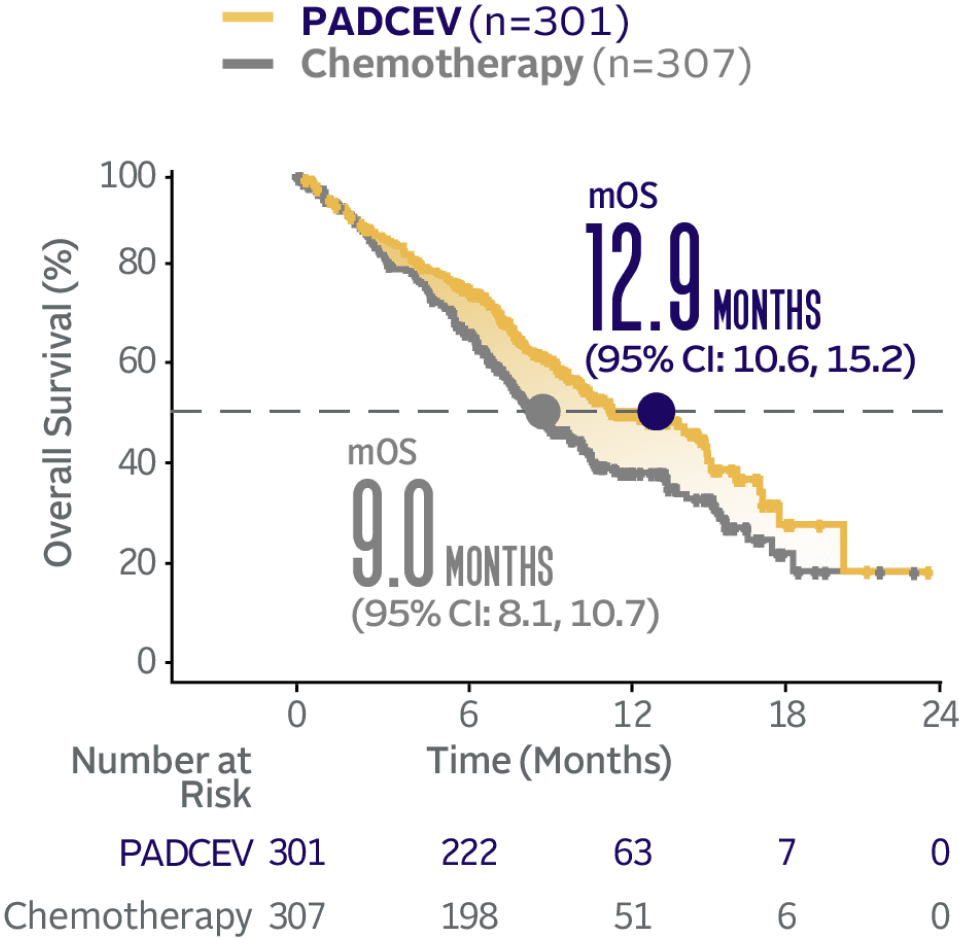
NEJM on X: "In women with advanced endometrial cancer, the median progression-free survival was 7.2 months with lenvatinib plus pembrolizumab and 3.8 months with chemotherapy; the median overall survival was 18.3 months
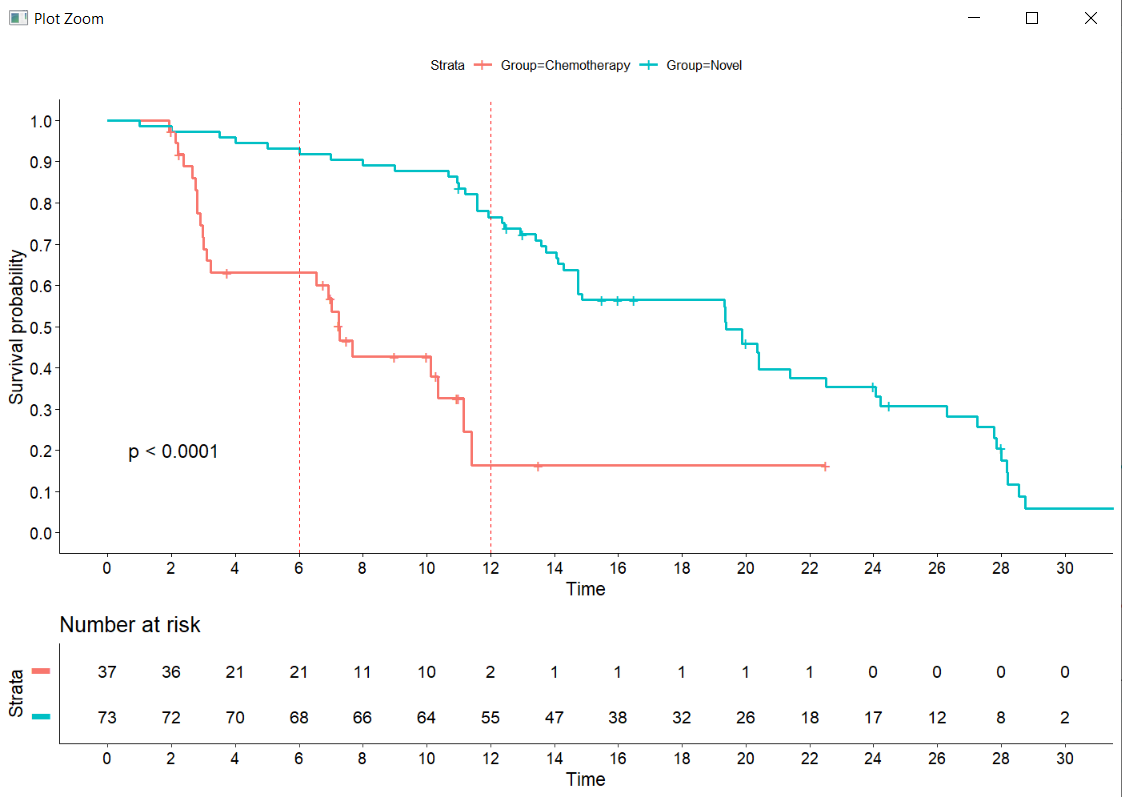
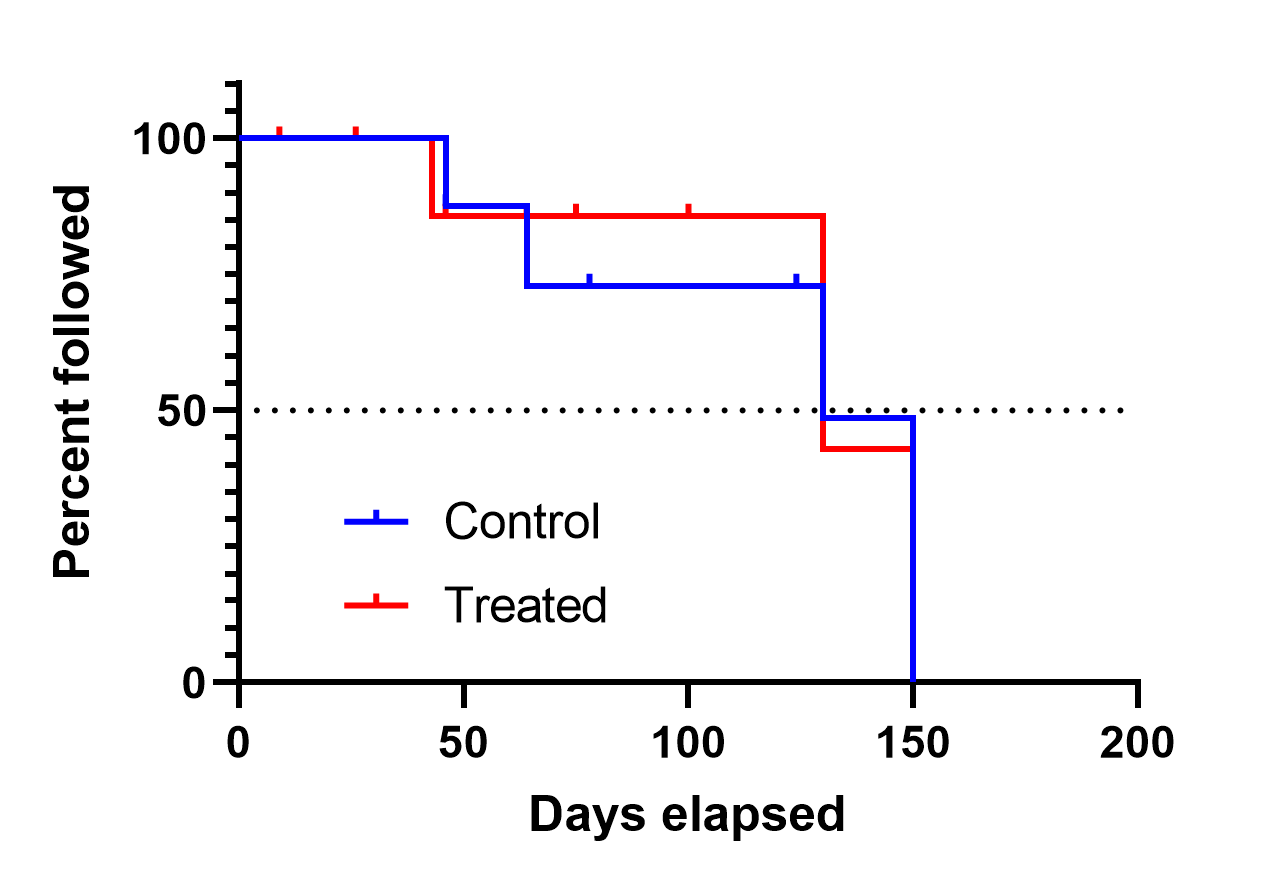
Interpreting Survival Probability in Relation to 'Number at Risk' and Median Follow-Up Times - interpret - Datamethods Discussion Forum
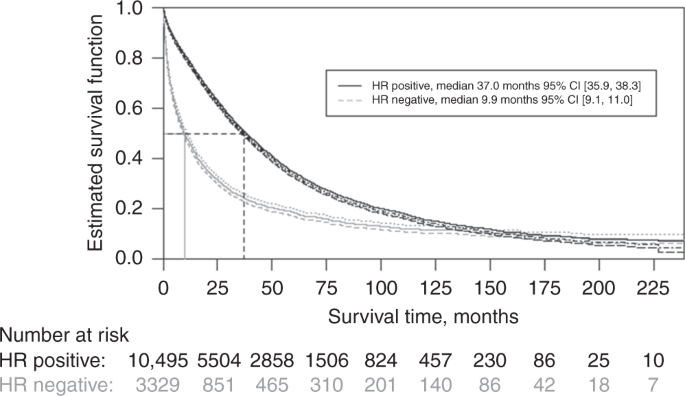
Overall survival of patients with metastatic breast cancer in Sweden: a nationwide study | British Journal of Cancer

Survival curves. (A) Overall survival (median 18.2 months). (B) PS of... | Download Scientific Diagram

Overall survival curve. Median overall survival time was 8.2 months.... | Download Scientific Diagram
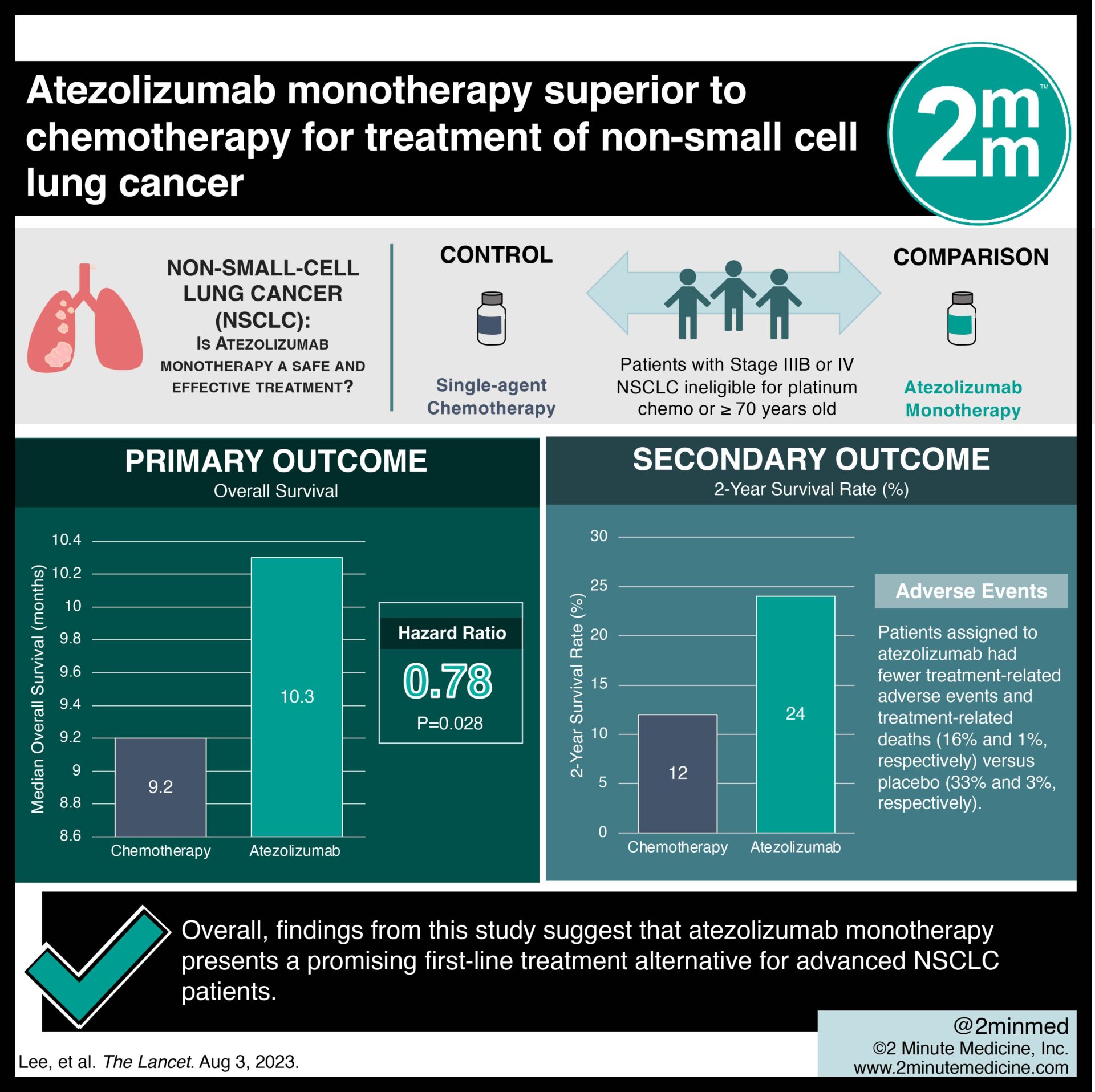
VisualAbstract: Atezolizumab monotherapy superior to chemotherapy for treatment of non-small cell lung cancer | 2 Minute Medicine

Prognostic Factors for Gastric Cancer Patients With One Stage IV Factor who Underwent Conversion Surgery | Anticancer Research
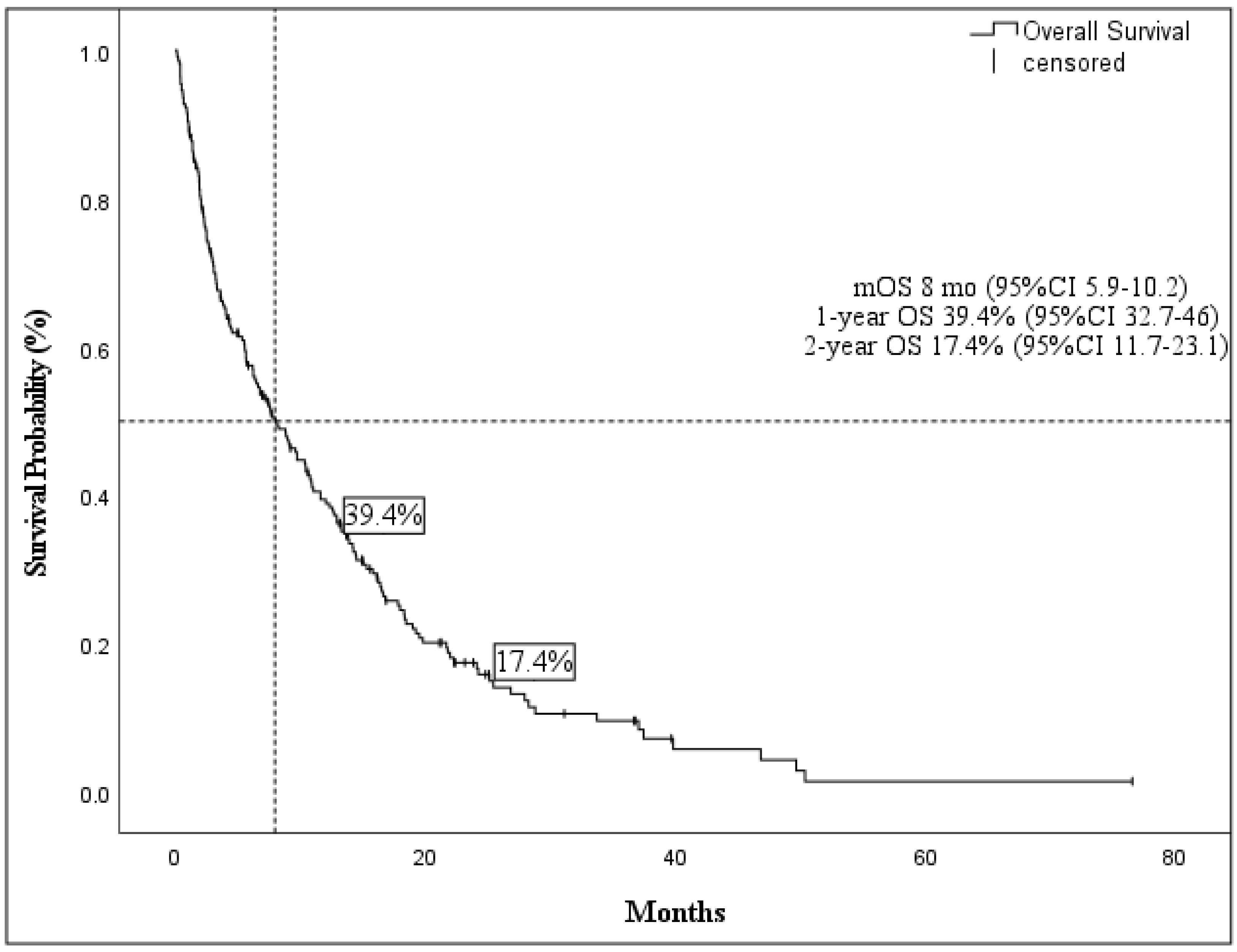
Cancers | Free Full-Text | Identification of Predictive Factors for Overall Survival and Response during Hypomethylating Treatment in Very Elderly (≥75 Years) Acute Myeloid Leukemia Patients: A Multicenter Real-Life Experience
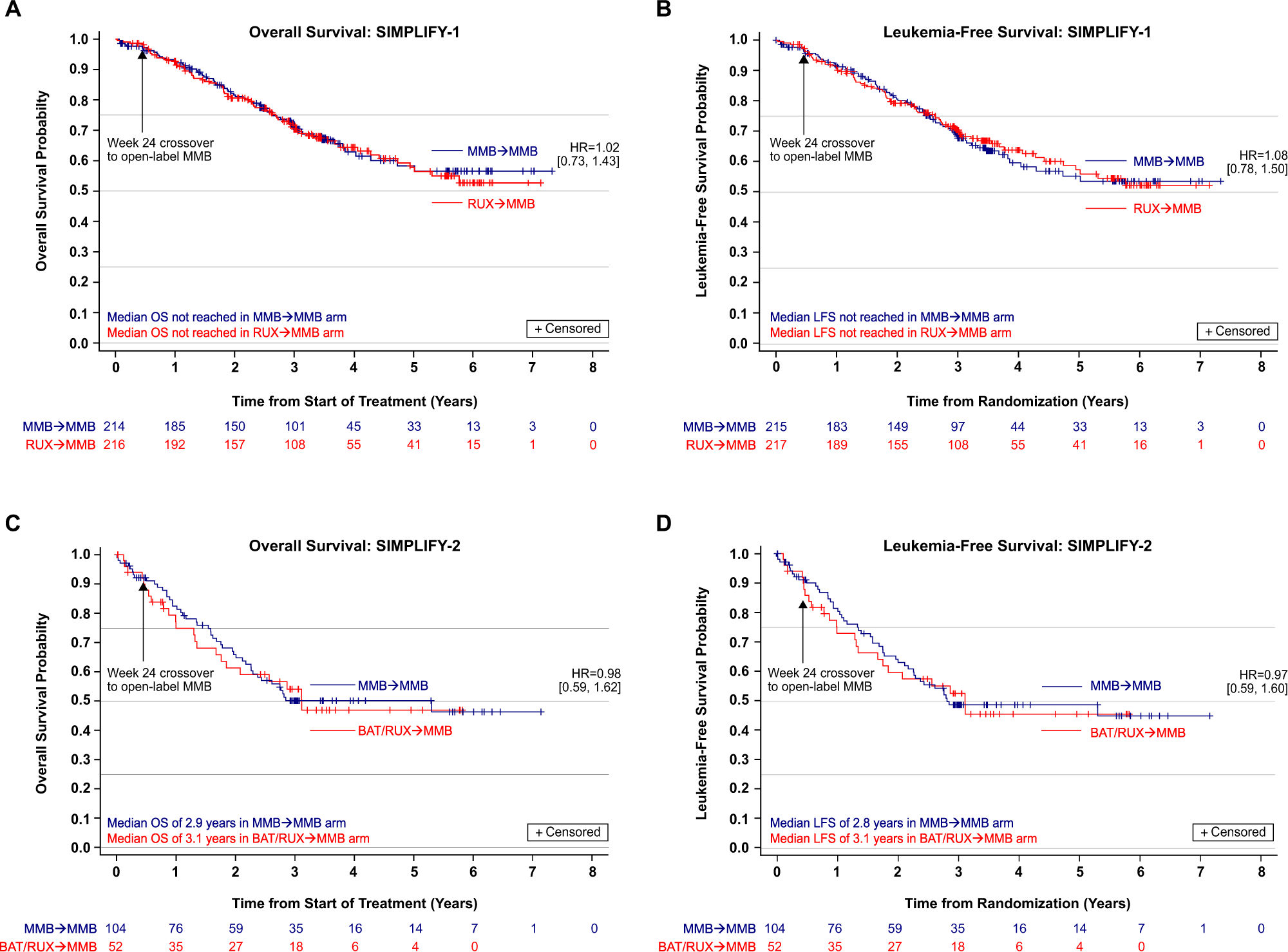
Overall survival in the SIMPLIFY-1 and SIMPLIFY-2 phase 3 trials of momelotinib in patients with myelofibrosis | Leukemia
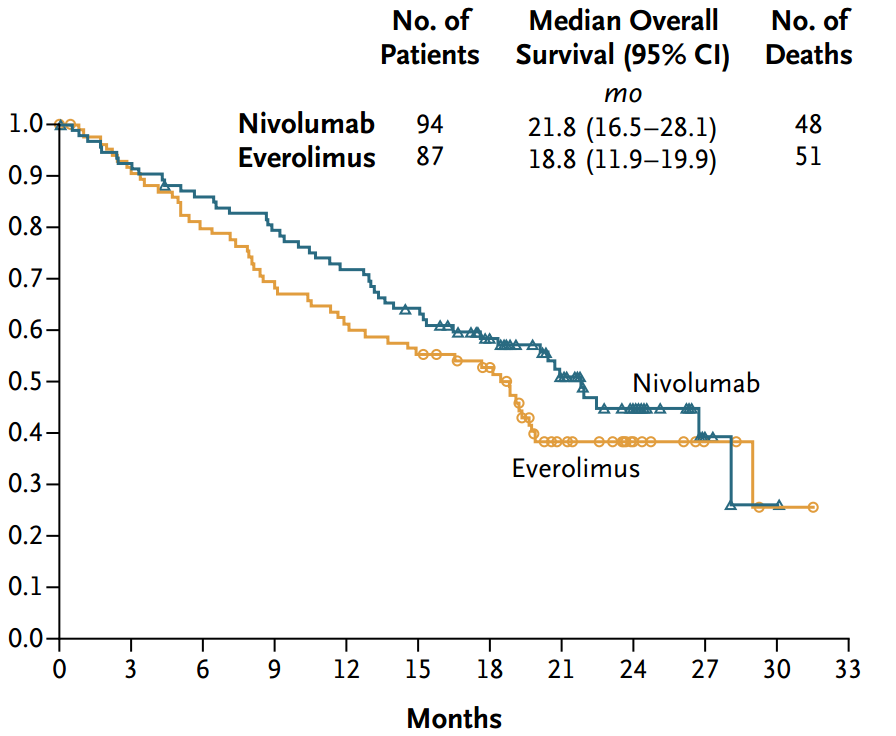
NEJM on X: "Nivolumab increases median overall survival by 5.4 months in adv renal cell cancer. #ECC2015 http://t.co/9DSZcnbnAs http://t.co/22qcFuEfzN" / X
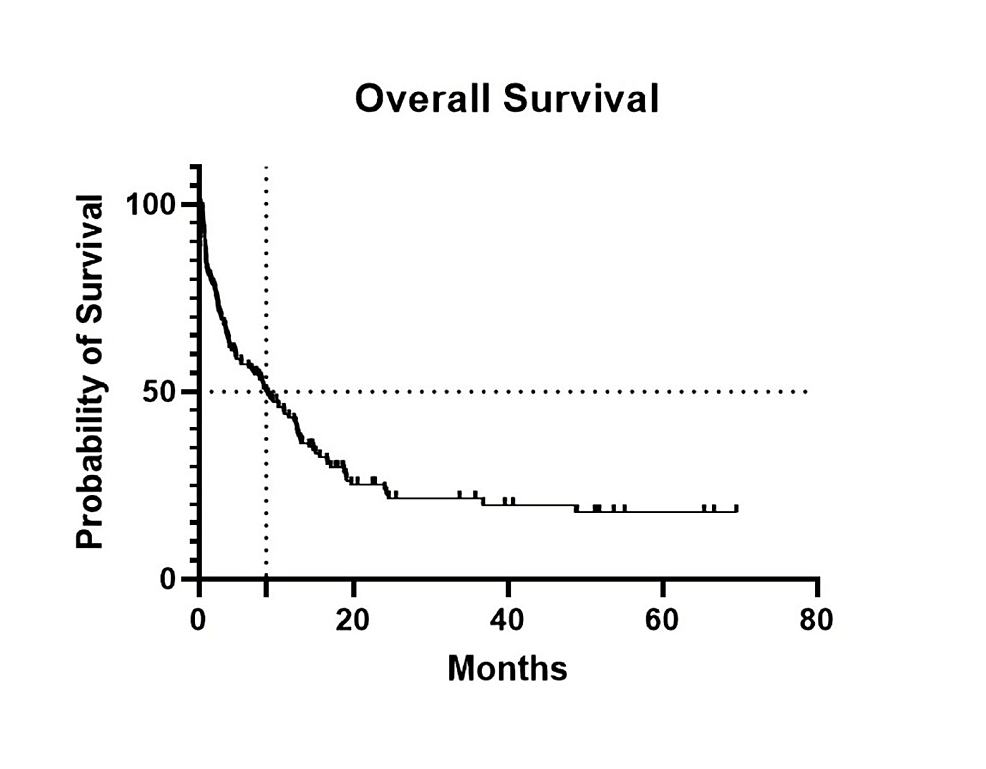
Cureus | A Survival Analysis of Acute Myeloid Leukemia Patients Treated With Intensive Chemotherapy: A Single Center Experience | Article

Progression free survival rate at 9 and 18 weeks predict overall survival in patients with malignant pleural mesothelioma: An individual patient pooled analysis of 10 European Organisation for Research and Treatment of
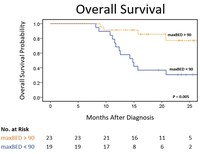
Early Clinical Data Suggests Nearly 2X Prolonged Median Survival for Inoperable, Locally Advanced Pancreatic Cancer with
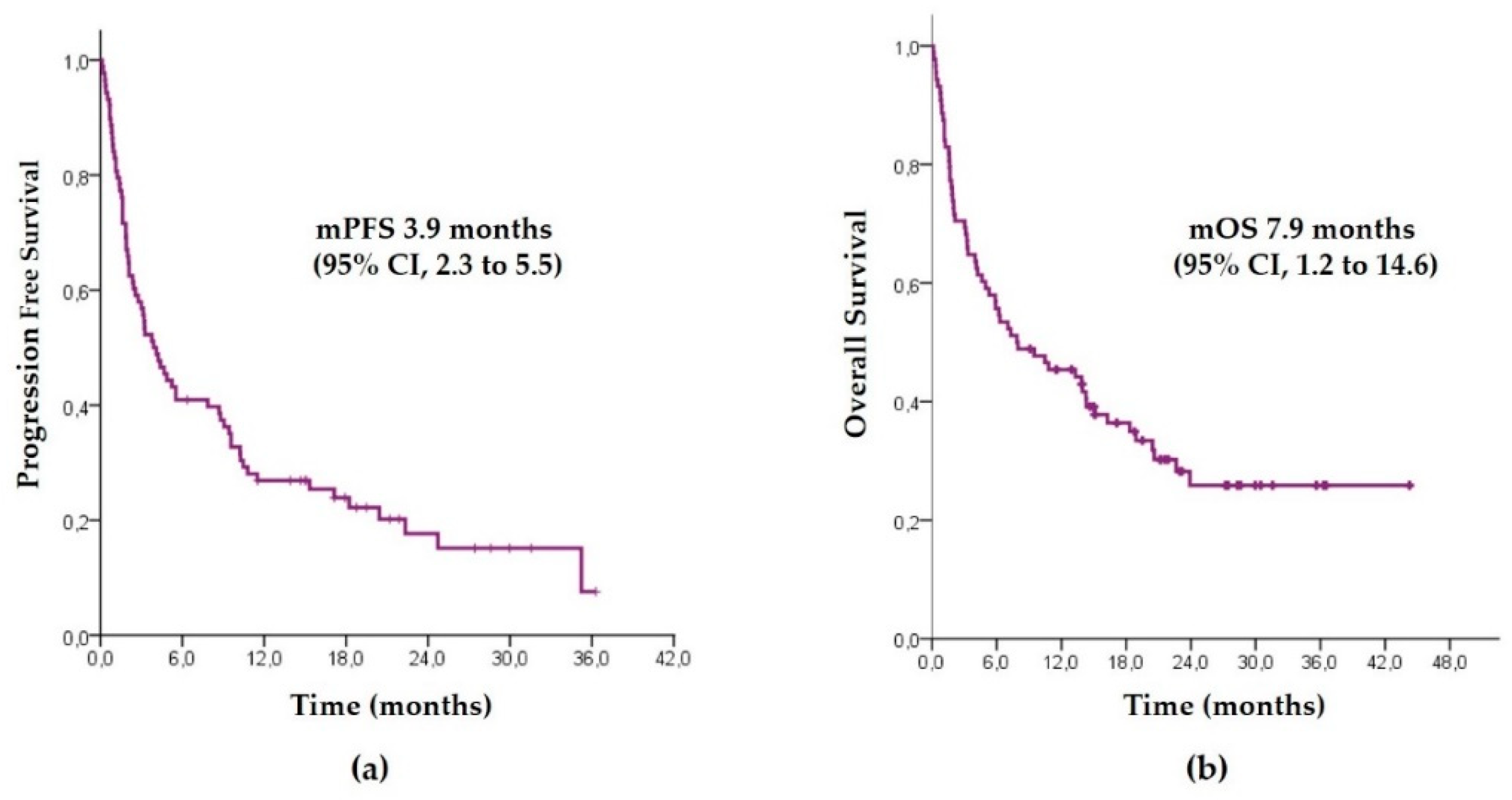
Biology | Free Full-Text | Influence of Performance Status on the Effectiveness of Pembrolizumab Monotherapy in First-Line for Advanced Non-Small-Cell Lung Cancer: Results in a Real-World Population